S2.2 The Covalent Model :
S2.2.1 Covalent Bonding, The Octet Rule and Lewis Formulas
📌 Covalent Bonding :
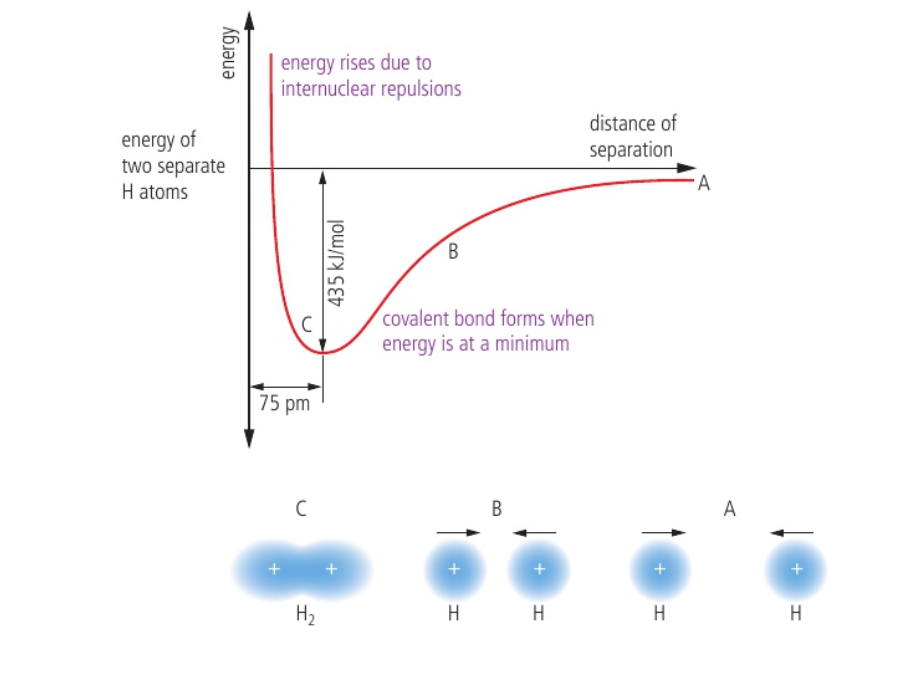
⭐️ A covalent bond is the electrostatic attraction between the positively charged nuclei of both atoms and the shared pair of electrons
- Non metals form covalent bonds as both atoms are trying to achieve noble gas configuration
- The shared pair of electrons is concentrated in the region between the two nuclei
- System will be stabilized when forces of attraction (nuclei and electrons) are balanced by forces of repulsion (nuclei and nuclei) – holds atoms at fixed distance apart
- Atoms with similar electronegativities tend to form covalent bonds
- Covalent bonds form at the point of lowest energy
📌 The Octet Rule
⭐️ Octet rule states that electrons will be shared so that the central atom has 8 electrons in its valence shell.
- The octet rule can be used to predict stable arrangements of atoms in covalent bonding
- Exception to octet rule – expanded/incomplete octets
- Limitation to octet rule – dealing with small atoms
- Lone pair/non-bonding pair are electrons in valence shell that are not involved in bonding
- Elements of group 18 (with the exception of Helium) have achieved octet configuration and hence display low reactivities and don’t form covalent bonds
📌 Lewis Formulas
- Lewis formulas shows all the valence electrons in a covalently bonded species
Step-by-Step Lewis Formula Drawing :
- Calculate total number of valence electrons by multiplying valence of each element by number of each time and totalling these
- Identify the skeletal structure of the molecule
- Central atom is always the least electronegative
- Starting with outer atoms, add electrons to complete shells
- Check against total number of valence – add multiple bonds if required
🧠 You can represent electron pairs using dots, crosses or lines. Be prepared to recognize all notations.
🧠 Paper 2 Tip : When drawing Lewis formulas for ions remember to add an electron for each negative charge and subtract one electron for each positive charge. Also remember to put the drawing in square brackets with the charge on the outside.
S2.2.2 Single, Double and Triple Covalent Bonds
- Sharing more than one pair of electrons results in a multiple covalent bond
- Single, double and triple bonds involve the sharing of one, two and three electron pairs, respectively
⭐️ The most abundant gas in air, N2, requires a triple covalent bond to complete the octets of both atoms.
📌 Bond Length and Bond Strength
- Short bonds are strong bonds
- Bond length is the distance between bonded nuclei
- Bond strength described in terms of bond enthalpy (enthalpy required to break the covalent bond)
- As atoms increase in size, bond strength decreases and bond length increases (as shared pair is held further from nucleus)
- Triple bonds are the shortest and strongest bonds, while single bonds are the weakest and longest
🧠 Note that the double bond is not twice as strong as the single bond, due to the fact that the bond contains a sigma element and a weaker phi element.
S2.2.3 Coordination Bonds
⭐️ A coordination bond is a covalent bond in which both electrons come from the same atoms
- Coordination bonds depicted with arrow, with head of arrow pointing to the origin of the two atoms
- Once they are formed, they are no different from coordination bonds
- Reaction between lewis acid and lewis base results in formation of a coordination bond
- Example of lewis acid-base reaction – formation of transition metal complexes
⭐️ Coordination bonds are also called coordinate covalent bonds or dative covalent bonds
🧠 If N forms four bonds in a molecule/ion one of the bonds is almost certainly a coordination bond.
S2.2.4 The Valence Shell Electron Pair Repulsion (VSEPR) Model
⭐️ VSEPR Model predicts shapes of molecules based on repulsion of electron domains around a central atom
- Electron domains (in valence of central atom) repel each other and hence take up positions to minimise these repulsions
- Electron domain refers to all electron locations in the valence shell whether they are occupied by non bonding electrons, single, double, or triple bonds
- The total number of electron domains around the central atom determine the geometrical arrangement of electron domains
- Electron domain geometry refers to how electron domains are arranged in space around the central atom
- Molecular geometry considers only the bonded electrons
- Non bonding pairs/lone pairs and multiple bonds cause more repulsion due to higher concentration of charge
- Non bonding pairs have a higher concentration of charge because they are not shared between two atoms
- Multiple bonds have a higher concentration of charge because they contain multiple electrons
- Repulsive forces decrease in the following order : lone pair-lone pair > lone pair – bonding > bonding – bonding
🔍 TOK connect : Sometimes, knowledge is easier to understand when it is simplified through a model. Does this suggest different qualities to the knowledge we acquire in different ways?
📌 VSEPR Geometry for Different Number of Electron Domains
| No. of Electron Domains | Electron Domain Geometry | No. of lone pairs | Molecular Geometry | Bond Angles | Example |
| 2 | Linear | 0 | Linear | 180° | CO2 |
| 3 | Triangular planar | 0 | Triangular planar | 120° | BF3 |
| 3 | Triangular planar | 1 | V-shaped | <120° | SO2 |
| 4 | Tetrahedral | 0 | Tetrahedral | 109.5° | CH4 |
| 4 | Tetrahedral | 1 | Trigonal pyramidal | ≈107° | NH3 |
| 4 | Tetrahedral | 2 | V-shaped | ≈104.5° | H2O |
| 5 | Triangular bipyramidal | 0 | Triangular bipyramidal | 90°, 120° | PCl5 |
| 5 | Triangular bipyramidal | 1 | See-saw | <90°, <120° | SF4 |
| 5 | Triangular bipyramidal | 2 | T-shaped | <90° | ClF3 |
| 5 | Triangular bipyramidal | 3 | Linear | 180° | I3– |
| 6 | Octahedral | 0 | Octahedral | 90° | SF6 |
| 6 | Octahedral | 1 | Square pyramidal | <90° | BrF5 |
| 6 | Octahedral | 2 | Square planar | 90° | XeF4 |
📌 Steps in determining shape of a molecule :
- Draw the Lewis formula for the molecule
- Count the total number of electron domains on the central atom
- Determine the electron domain geometry using the table above
- Determine the molecular geometry based on the number of lone pairs
- Adjust the bond angles accordingly
S2.2.5 Bond Polarity
⭐️ Bond polarity results from the difference in electronegativities of the bonded atoms
⭐️ Electronegativity – measure of attraction of an atom in a molecule for the electron pair in the covalent bond of which it is a part
- In a covalent bond between different atoms – electron pair is not shared equally
- Polarity results from unequal sharing
- Asymmetrical distribution of electron density forms a bond dipole (eg. in HF : F is more electronegative and gains a partially negative charge)
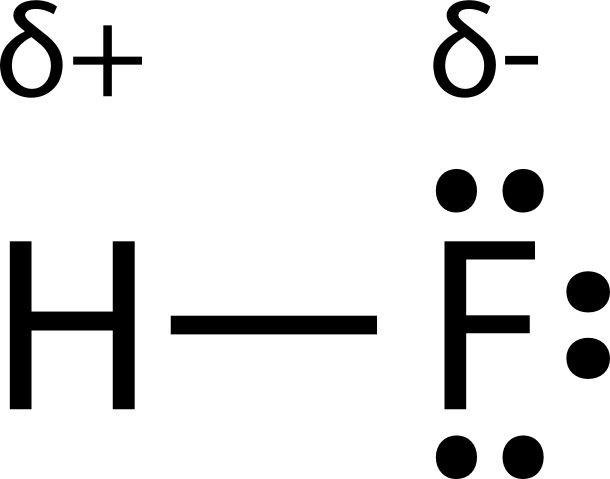
- Bond dipole is used to show partially separate and opposite charges in a bond
- Extent of polarity depends on extent of difference in electronegativities
📌 Pauling Scale of Electronegativity :
- Non metals have higher electronegativity than metals
- Electronegativity decreases down a group
- Electronegativity increases across a period
- F, O, Cl, N are the most electronegative elements
- Pauling scale of electronegativity can be used to measure the relative electronegativity of different elements
- Atoms with similar electronegativities will form covalent bonds
- Atoms with vastly different electronegativities will form ionic bonds
🧠 Exam Tip : To remember trends in electronegativity, just remember that Fluorine is the atom with the highest electronegativity. Hence electronegativity must decrease down a group (away from Fluorine) and increase across a group (towards Fluorine).
⭐️ Most noble gases do not have electronegativity values because they do not generally form compounds. Xenon does form a variety of compounds and hence is assigned an electronegativity value of 2.6
- Difference of 1.7 corresponds to 50% ionic character
- <1.7 difference = covalent bonding
- Truly non polar bonds – diatomic molecules (eg. H2, F2) as electronegativity difference is 0
- Referred to as pure covalent bonds
- Partial separation of charges induces some level of ionic character
- Polar bonds considered to be intermediates between pure covalent and pure ionic bonds – idea of bonding continuum
S2.2.6 Molecule Polarity
⭐️ Molecular polarity depends on both bond polarity and molecular geometry
- Bond polarity refers to the charge separation between bonded atoms
- Even with polar bonds, a molecule can be non polar if the dipoles cancel out
- For a molecule to be polar – opposite ends must have slight charges
- If bonds of equal polarity are symmetrically arranged, the charges will cancel out leading to a non polar molecule
- Net dipole (which results in molecular polarity) will occur if either bonds have different polarities or are asymmetrically arranged
S2.2.7 Covalent Network Structures
- Substances that exist as networks in the solid state, no individual molecules
- Crystalline lattice in which atoms are linked through covalent bonds
- Allotropes have different structural and bonding properties of the same element in the same physical state so have different physical and chemical properties as well
📌 Allotropes of Carbon
- Diamond
- sp3 hybridised (covalently bonded to 4 others)
- Tetrahedral arrangement, with a bond angle of 109.5
- Non-conductor of electricity due to non-mobile electrons
- Very efficient thermal conductor
- Used for jewellery, cutting glass
- Very high boiling point (4000 C) and melting point
- Hard and lustrous
🧠 Exam Tip : Remember the structure of diamond is a covalent network. Tetrahedral is the arrangement of atoms around each carbon and not the name of the structure.
- Graphite
- sp2 hybridised (covalently bonded to 3 others)
- Hexagons in parallel layers, approximately 120
- Weak LDF between layers
- Good conductor of electricity – delocalised electrons free to move parallelly across layers
- However, electrons are not free to move between layers so graphite is an electrical insulator perpendicular to the plane of layers
- Not a thermal conductor unless direction of heat is parallel to crystal layers
- Non lustrous, grey crystalline solid
- Soft, slippery, brittle
- Very high melting point
- Most stable allotrope of carbon
- Used in pencils, electrodes
- Graphene
- sp2 hybridised (covalently bonded to 3 others)
- Single layer of hexagons, 120
- Good electrical conductor (C forms only 3 bonds)
- Best thermal conductivity
- Almost transparent
- Thinnest, strongest, flexible
- High melting point
- Used in transmission electron microscopy, electronic devices
🔍TOK Connect : Will the potential of graphene be realized and lead to innovations and applications? What is the role that imagination plays in directing the focus of research conducted by scientists?
- C60 Buckminister Fullerene
- Not a giant molecule as it has a fixed formula
- sp2 hybridised, sphere (60 C)
- Poor conductor of electricity, low thermal conductivity
- Low melting point as it doesn’t have a network structure
- Light and strong
- Used in medical devices and nanotubes
- Silicon
- Giant lattice structure in tetrahedral array
- Silicon covalently bonds with Oxygen (Si-Si has a greater bond length than C-C, and weaker bond strength)
- Silicon Dioxide
- Covalent network structure
- Each Si bonded to 4 O in a tetrahedral arrangement
- Each O bonded to 2 Si atoms (bent)
S2.2.8 and S2.2.9 Intermolecular Forces
- Attractive forces between molecules
- Intermolecular forces depend on size and polarity of molecules
- Strength of intermolecular forces influences physical properties
- London Dispersion Forces
- Only force present in non polar molecules
- Weaker than covalent bonds
- Present between all molecules in solid/liquid state
- Constant motion and asymmetrical distribution (cloud of negative charge) lead to the formation of temporary and instantaneous dipoles – constantly appearing and disappearing
- Instantaneous dipole induced dipole interactions
- Gets stronger as relative molecular mass increases (increase in number of electrons)
- LDF stronger in aromatic compounds
🧠 Exam Tip : When asked to name forces between molecules, LDF must always be mentioned, regardless of other, stronger forces that might be present.
- Dipole-Dipole Attraction
- Permanent separation of charges based on difference in electroegativity
- Present in polar molecules
- Permanent dipole generates force called dipole-dipole attraction
- If molecules with similar molecular masses compared – polar molecules have higher boiling points due to permanent dipole-dipole interactions
- Dipole-induced Dipole Forces
- Permanent dipole of polar molecule can induce separation of charge (temporary dipole) on non polar molecules
- Mixture of polar and non polar molecules
⭐️ Vanderwaal’s forces – LDF, dipole-dipole, dipole-induced dipole – all forces that do not involve electrostatic attractions between ions or bond formation.
- Hydrogen bonding
- When N/O/F joined to an H atom – large electronegativity difference withdraws electron density from H
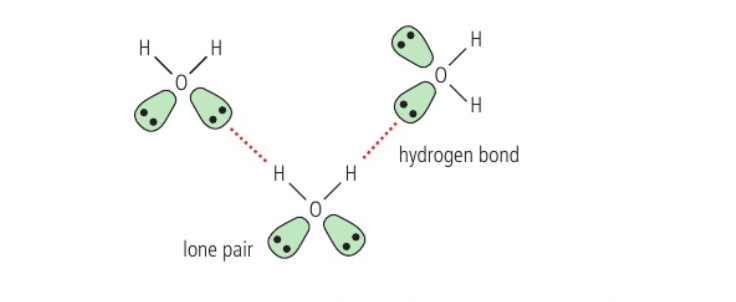
- H δ
+ exerts strong attractive force on δ
– in neighbouring molecule
- Strongest intermolecular force – causes boiling points to be significantly higher than predicted from molar mass
- Hydrogen bonding allows for ice to be less dense than water and still hold its shape.
⭐️ If it wasn’t for hydrogen bonding, H2O would be a gas at room temperature
📌 Physical Properties of Covalent Compounds
- Melting and boiling points
- Energy supplied to break intermolecular bond depends on strength of ong
- Substances with higher relative molecular masses have higher melting and boiling points due to LDF
- When comparing substances of similar molecular masses – consider the total of all intermolecular forces present
- Volatility
- Tendency of a substance to vaporize
- Stronger IMF, less tendency to vaporize
- Solubility
- Substances will dissolve in a solvent if IMF of substance and solvent are similar
- How much energy is needed to over come IMF in solute and solvent is paid back by how much energy is released due to the IMF formed between solute and solvent
- Substances with hydrogen bonding are soluble in water
- Longer chain alcohols become less soluble in water (polar part is small compared to long hydrocarbon chain)
- Giant molecular structures are generally insoluble in most solvents
- Electrical conductivity
- Covalent compounds do not contain ions and hence do not conduct electricity
- In the event that polar covalent compounds ionize they can conduct electricity to a certain degree
- Some giant structures are good conductors of electricity (graphene, graphite)
S2.2.10 Intermolecular Forces and Chromatography
- Chromatography is a means of separating the components of a mixture
- Components of mixture as separated based on affinity for stationary and mobile phase
- Thin layer chromatography
- Stationary phase is a plate coated in silica gel/aluminium – can H bond with components of mixture
- Particles are adsorbed based on affinity ( formation of IMF between silica surface and solute molecules) – stay on surface
- More polar molecules form stronger interactions with silica surface
- Faster, and can be regulated better
- Paper chromatography
- Stationary phase – water coated fibres in paper
- Solvent – mobile phase
- Components soluble in stationary phase move slower
- Components soluble in mobile phase move faster

- Partition is the tendency of a solute to be distributed between two immiscible solvents according to its solubility in each
- RF value close to 1 – high solubility in mobile phase
- Low RF – high affinity for stationary phase
S2.2.11 Resonance Structures [ HL]
- Resonance structures occur when there is more than one possible position for a double bond in a molecule
- Delocalisation – sharing of a pair of electrons between 3 or more atoms
- Delocalised electrons spread out offering more stability to the atom
- Eg : Ozone (O3)
- Lewis structure shows one O-O single bond and one O-O double bond – would expect that it has two bonds that that differ in length and strength
- Ozone actually has two equal bonds, intermediate in length and strength between double and single bonds
- True structure is a resonance hybrid of both structures (both positions of the double bond)
- Resonance structures contribute equally to resonance hybrid
- Resonance hybrid does not mean the structure flips between the two options, rather it is an intermediate of both options
🧠 Paper 2 Tip : This can be used explain why O2 requires a shorter wavelength (higher energy)light to break, as the double bond is stronger than the hybrid present in O3.
🌍 Real World Connect : O2 and O3 play important roles in protecting the surface of the Earth from UV radiation. The formulas for constant formation and decomposition are as follows :
Formation :
O2(g) → 2 O*(g)
O*+O2→O3
Decomposition :
O3(g) → O*+O2
O3 + O*→ 2 O2
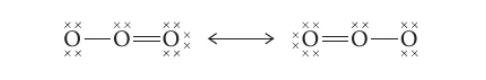
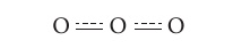
- If a structure can be accurately described by only changing position of double bonds/lone pairs and everything else remains the same, then a resonance structure is possible
- Bond order is a term used to describe the strength of bonds
- Resonance structures usually have fractional bond orders (between that of a single, double or triple bond)
🧠 Paper 2 Tip : We do not draw lone pairs of electrons in resonance hybrid diagrams as they are involved in the process of delocalisation and do not have fixed positions.
S2.2.12 Benzene [HL]
- Benzene ( C6H6) is an important example of a molecule that has resonance – different possible locations for C=C bond
- 6C in a hexagonal ring with trigonal planar arrangement
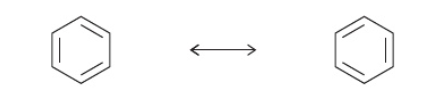
- Resonance hybrid of benzene (true structure) is represented by a hexagon with a circle inside.
🧠 Paper 2 Tip : When drawing the structure of Benzene, make sure you don’t forget to include the ring inside the hexagon. Otherwise, the structure will represent a different cyclohexane, C6H12
- Kekulé structure of benzene explained some known properties of Benzene like the fact that it has no isomers, but didn’t explain the high reactivity for such an unsaturated molecule
- Technological advancements helped develop the current model of benzene structure
📌 Evidence for Kekulé structure of Benzene
- All C=C bonds are equal in length (between single and double bonds)
- Less energy than predicted given out in reactions with hydrogen
- Benzene has no isomers and does not undergo addition reactions
- Only 3 isomers of dibromobenzene exist
🔍TOK Connect : It is said that Kekulé visualized the structure of benzene following a dream about snakes biting each others tails. To what extent can imagination and unconscious processes be important in scientific advancement?
S2.2.13 Molecules with Expanded Octet [HL]
- While the octet arrangement is the most common, some elements can form molecules with incomplete/ expanded octets
- Small atoms like B or Be can form stable molecules with fewer than an octet
- When the central atom is from period 3 and above, sometimes, it can form an extended octet with more than 8 valence
- This occurs because the d subshell is close in energy to the p subshell and electrons can be promoted (eg from 3p to 3d) to form additional electron pairs (eg. PF4)
⭐️ Molecular and electron domain geometries for species with expanded octets are given in the table above (see S2.2.4)
S2.2.14 Formal Charge [HL]
⭐️ Formal charge is the charge that an atom would have if we assume that all electrons in the covalent bonds are shared equally (ie. all atoms have the same electronegativity)
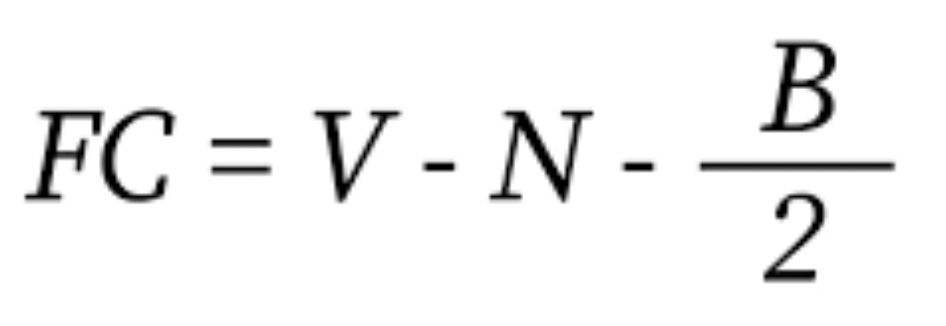
V = valence electrons in uncombined atom
N = no of non bonding electrons
B = no of bonding electrons
⭐️ Preferred lewis structure is the one with FCs closest to 0
- Formal charge can be used to predict a preferred Lewis formula
- Equivalent lewis structures are those that have the same numbers of single, double and triple bonds (eg. resonance structures)
- Non equivalent structures contain different numbers of single, double and triple bonds
- FC can be used to compare stabilities of non-equivalent structures only
- Sum of FCs for neutral ion = 0
- Sum of FCs for charged ion = charge
📌Assumptions of FC :
- Each atom has equal share of a bonding electron in a bond (even if it is a coordination bond)
- All atoms own their lone pairs entirely
S2.2.15 Sigma and Pi Bonds [HL]
- Bond forms when two atomic orbitals, each containing 1 electron, combine to form a new molecular orbital that is at a lower energy level
📌 Sigma Bond :
⭐️ Sigma bonds (σ) form by the head on combination of atomic orbitals where the electron density is concentrated along the bond axis
- Head-on overlap along bond axis
- Can occur between s orbitals, p orbitals, or hybrid orbitals
- All single covalent bonds are sigma bonds
📌Pi Bond :
⭐️ Pi bonds (π) form by the lateral combination of p orbitals where the electron density is concentrated on opposite sides of the bond axis
- Sideways overlap
- Occurs between p orbitals
- Occurs in double or triple bonds
- Pi bonds are weaker than sigma bonds as electron density is further away from the positively charged nucleus
- Double bond
- 1 sigma bond
- 1 pi bond
- Triple bond
- 1 sigma bond
- 2 pi bonds
S2.2.16 Hybridisation [HL]
- Hybridisation is the concept of mixing atomic orbitals to form new hybrid orbitals for bonding
- Carbon in the ground state has an electronic configuration of 1s22s22px12py1 with 2 singly occupied orbitals available for bonding
- This means it undergoes changes to its configuration to be able to form 4 covalent bonds
- A process called excitation occurs, promoting one 2s electron to the empty 2p orbital, creating 4 singly occupied orbitals available for bonding
- The amount of energy required to promote is paid back by the energy released by formation of 4 bonds
- The orbitals are hybridised to form 4 equal in energy sp3 orbitals so that all bonds are the same
- sp3 hybridisation
- 1s and 3p orbitals
- When carbon forms 4 single bonds
- Tetrahedrally arranged at 109.5
- Overlap of hybrid orbitals with orbitals of any other atom form 4 sigma bonds
- sp2 hybridisation
- 1s and 2p orbitals
- When carbon forms a double bond
- Form a triangular planar shape at 120
- Overlap with neighbouring orbitals forms 3 sigma bonds
- Two carbon atoms with p orbital that didnt take part in hybridisation form a pi bond
- sp hybridisation
- 1s and 1p orbital
- When carbon forms a triple bond
- Orient at 180 with a linear shape
- 2 unhybridized p orbitals form 2 pi bonds
📌Hybridization in Benzene :
- Each C is sp2 hybridised and forms 3 sigma bonds, leaving one unhybridised p orbital
- These orbitals overlap from both sides forming a delocalized π electron cloud with electron density concentrated in 2 donut shaped rings above and below the equatorial plane
🔍TOK Connect : Electron orbitals do not exist as physical entities but their description as a volume of space provides a convenient basis to explain bonding and electron properties. Hybridization is explained as a mixing process, for better understanding of bonding, but is in reality based on complex mathematical manipulation.To what extent does this use of language and imagery affect our understanding of what is truly being represented?