R1.2.2 – Hess’s Law
📌 What is Hess’s law?
- Hess’s Law refers to the law of conservation of energy within a chemical reaction
- The law suggests that regardless of the pathway of a chemical reaction, the same amount of energy is used and produced during the course of the reaction
- This can be summarised as : the enthalpy change for any reaction is independent of the route provided the same starting conditions
- The following diagram depicts how a ‘cycle’ can be created from this law
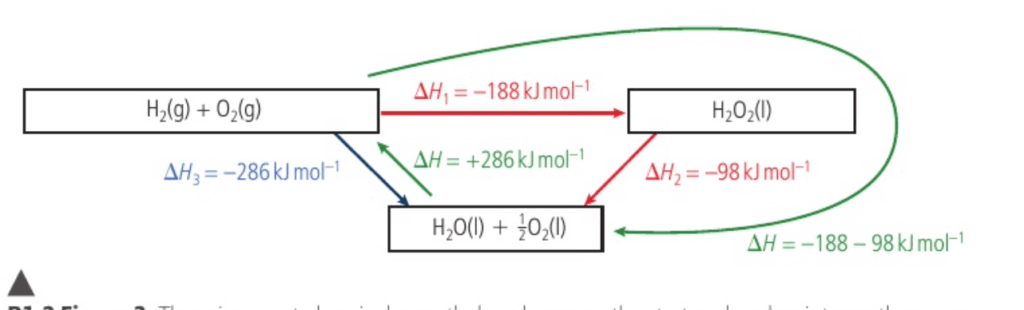
- We can see that H1 (-188) is equivilaent to H3 (-286) + H2(+98)
⭐️ it is important to keep in mind that the sign for the enthalpy change will switch depending on the direction of the reaction
📌 Using Hess’s Law
- Hess’s Law allows us to calculate the enthalpy change for reactions where it cannot be directly measured in the laboratory
- If two elements or compounds do not directly combine to form a product, the enthalpy change of this reaction can be measured by using Hess’s law on the intermediary products formed during the reaction
- Hess’s Law can be summarised as the following equation :
ΔH =
ΔH1 + ΔH2 + ΔH3 … where ΔH1, ΔH2 and so on refer to the enthalpy changes for the reactions producing intermediate products