R1.2.1 – Bond enthalpy
📌 Breaking bonds
- Breaking bonds in an endothermic process
- When bonds are broken, energy is absorbed from the surroundings into the system in order to provide sufficient energy for bond breaking, thus making it endothermic
- Bond en thalpy is defined as the amount of energy required to break one mole of bonds in a gaseous substance under STP
- To calculate the bond enthalpy in molecules containing more than 2 atoms (eg H2O), the average bond enthalpy of the breaking of the two bonds is taken (see example below)
Example :
H2O (g) → OH(g) + H(g) requires +502kJ mol-1
OH (g) → O(g) +H(g) requires +427 kJ mol-1
Therefore, the average bond enthalpy for the O-H bond is (502+427)/2 = 464.5 kJ mol-1
- Bond enthalpies do not involve the energy used to break intermolecular forces
⭐️ values for average bond enthalpies can be found in sections 13&14 of the data booklet
📌 Polarity
- Polar bonds between two atoms of two different elements are generally stronger than non-polar bonds between atoms of the same element
- Bond enthalpies can be used to calculate the electronegativity of an element
- Average bond enthalpy decreases as electronegativity between 2 atoms decreases
📌 Making bonds
- Bond making is an exothermic process
- When new bonds are formed in a compound, they process releases energy into the surroundings
- The same amount of energy needed to break a bond is given out when making the bond. However since it is an exothermic reaction, the enthalpy change will be negative.
Example :
If :
H2(g)→H2(g) H(g) +H(g) ΔH = +436kJ mol-1
then :
H(g) +H(g) →H2(g) ΔH = -436kJ mol-1
📌 Calculating enthalpy
- When calculating bond enthalpy, you must first use known (standardised) values to calculate the energy absorbed (breaking) and the energy produced (breaking)
- This calculation can be done by subtracting the enthalpy of the bonds formed in the products from the enthalpy of bonds broken in the reactants
- This can be summarised in the following equation :
ΔHreaction = ∑ E(broken) – ∑ E(formed)
- The following example shows this can be calculated
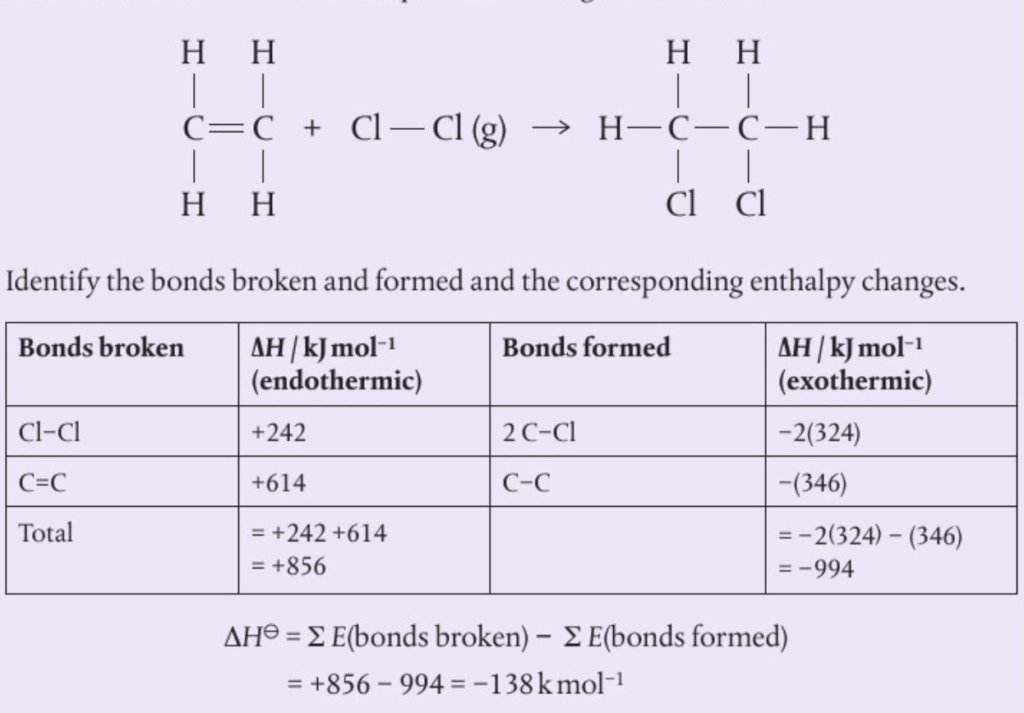
🧠 you are not required to know the values for any enthalpies, they are available in the data booklet