B1.1.3 – LIPIDS: STRUCTURE, TYPES & FUNCTIONS
📌Definition Table
| Term | Definition |
|---|---|
| Lipid | Hydrophobic organic molecule including fats, oils, waxes, steroids, and phospholipids. |
| Triglyceride | Lipid formed from glycerol and three fatty acids. |
| Fatty Acid | Hydrocarbon chain with a carboxyl group; may be saturated or unsaturated. |
| Saturated Fatty Acid | Fatty acid with no double bonds between carbon atoms. |
| Unsaturated Fatty Acid | Fatty acid with one or more double bonds between carbon atoms. |
| Phospholipid | Lipid with two fatty acids and a phosphate group attached to glycerol; amphipathic. |
📌Introduction
Lipids are a diverse group of hydrophobic molecules that play key roles in energy storage, membrane structure, insulation, and signalling. Their nonpolar nature makes them insoluble in water but soluble in nonpolar solvents. Lipids are not true polymers, as they are not composed of repeating monomers, but they are built from smaller units like glycerol and fatty acids.
❤️ CAS Link: Create a dietary awareness campaign showing the health effects of different fats, including omega-3 benefits and risks of trans fats.
📌 Structure and Types of Lipids
- Triglycerides: Glycerol + three fatty acids linked via ester bonds.
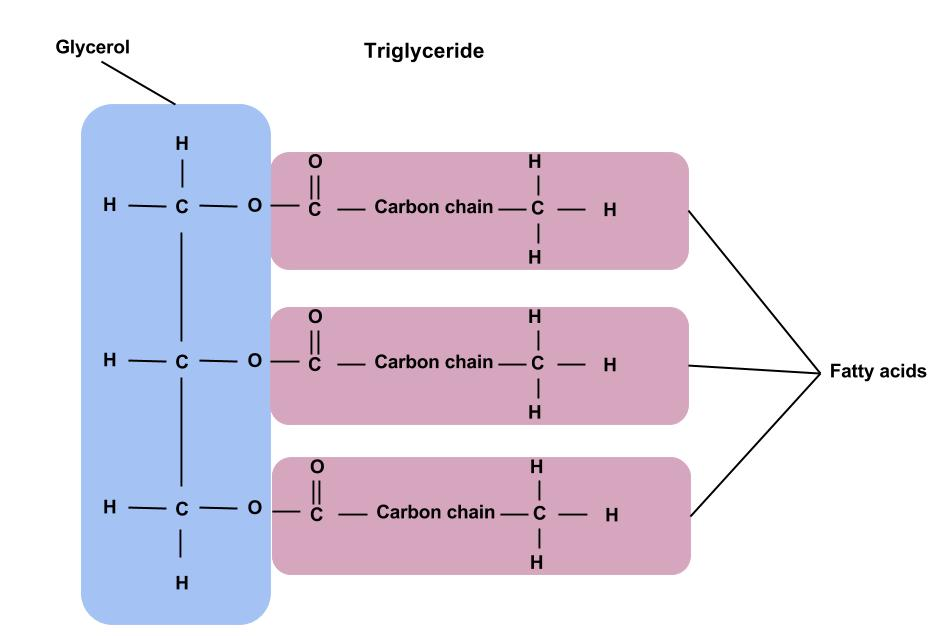
- Fatty acids can be saturated (straight chains, solid at room temp) or unsaturated (kinked chains, liquid at room temp).
- Monounsaturated: One double bond; Polyunsaturated: multiple double bonds.
- Phospholipids: Amphipathic molecules forming the bilayer of membranes.
- Steroids: Four fused carbon rings; include cholesterol, testosterone, and oestradiol.
- Waxes provide waterproofing in plants and animals.
🧠 Examiner Tip: Always specify that phospholipids are amphipathic, as this links structure directly to membrane function.
📌 Properties and Biological Roles
- High energy yield per gram — ideal for long-term energy storage.
- Insolubility in water prevents interference with osmotic balance.
- Provide insulation in animals (e.g., blubber in whales).
- Aid buoyancy in aquatic animals due to low density.
- Serve as precursors for hormones and signalling molecules.
- Contribute to waterproofing in plants (cuticle) and animals (feathers).
🌍 Real-World Connection: Polar bears rely on stored lipid reserves for insulation and energy during fasting periods in Arctic winters.
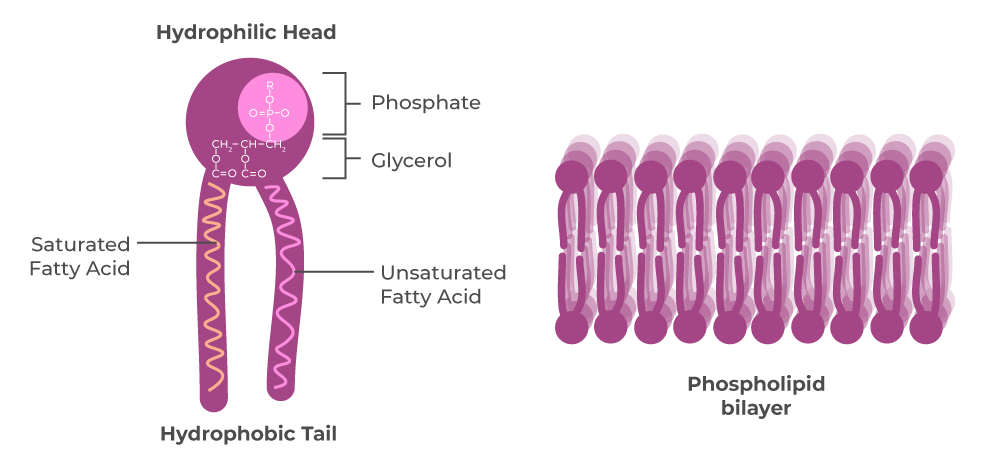
📌 Phospholipid Bilayer and Membranes
- Phospholipids arrange with hydrophobic tails inwards and hydrophilic heads outwards.
- Creates a selectively permeable barrier controlling entry and exit of substances.
- Allows diffusion of nonpolar molecules (O₂, CO₂, steroid hormones).
- Embedded proteins assist in transport of polar molecules and ions.
- Fluidity affected by fatty acid composition and cholesterol content.
- Essential for compartmentalisation of cellular processes.
🌐 EE Focus: An EE could test membrane permeability under different lipid compositions or temperature conditions.
📌 Health Implications of Lipids
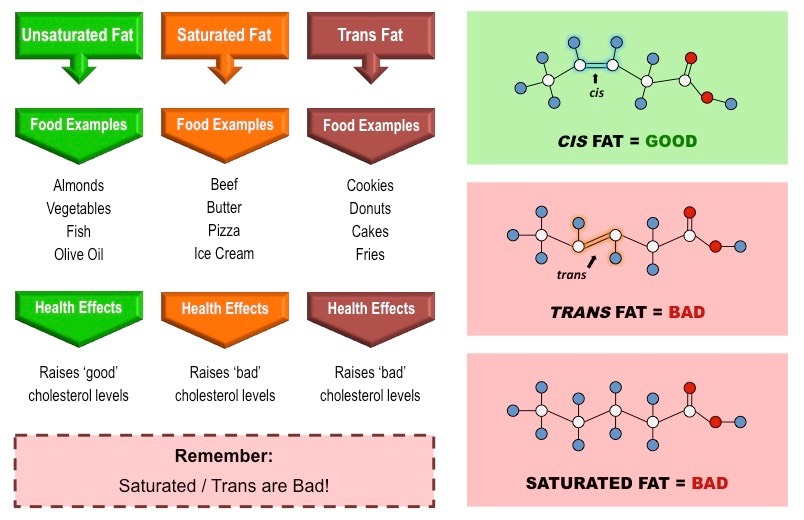
- Saturated fats: Linked to higher LDL cholesterol, risk of atherosclerosis.
- Unsaturated fats: Associated with heart health benefits (omega-3 fatty acids).
- Trans fats: Artificially hydrogenated; strongly linked to cardiovascular disease.
- Balance between omega-3 and omega-6 fatty acids influences inflammation responses.
- Dietary guidelines encourage reducing saturated and trans fat intake.
- Lipid profiles are a key health diagnostic tool.
🔍 TOK Perspective: How do cultural dietary traditions influence perceptions of “healthy” fats?