B2.3.1 – STEM CELLS AND POTENCY
📌Definition Table
| Term | Definition |
|---|---|
| Stem Cell | Undifferentiated cell capable of self-renewal and differentiation into specialised cell types. |
| Potency | The ability of a stem cell to differentiate into different cell types. |
| Totipotent | Stem cell that can form all cell types, including embryonic and extraembryonic tissues. |
| Pluripotent | Stem cell that can form all cell types of the body but not extraembryonic tissues. |
| Multipotent | Stem cell that can form a limited range of cell types related to a particular tissue or organ. |
| Unipotent | Stem cell that can only form one cell type but retains the ability to self-renew. |
| Stem Cell Niche | Microenvironment that maintains stem cell function and regulates differentiation. |
📌Introduction
Stem cells are unique in their ability to both self-renew and differentiate into specialised cell types. They play a crucial role in growth, development, and repair in multicellular organisms. Potency describes the range of cell types a stem cell can produce. Different levels of potency are observed throughout development, from the zygote’s totipotency to the restricted capabilities of adult stem cells.
❤️ CAS Link: Volunteer with a blood donation and marrow registry campaign to raise awareness about stem cell therapies and their role in treating blood cancers.
📌 Properties of Stem Cells
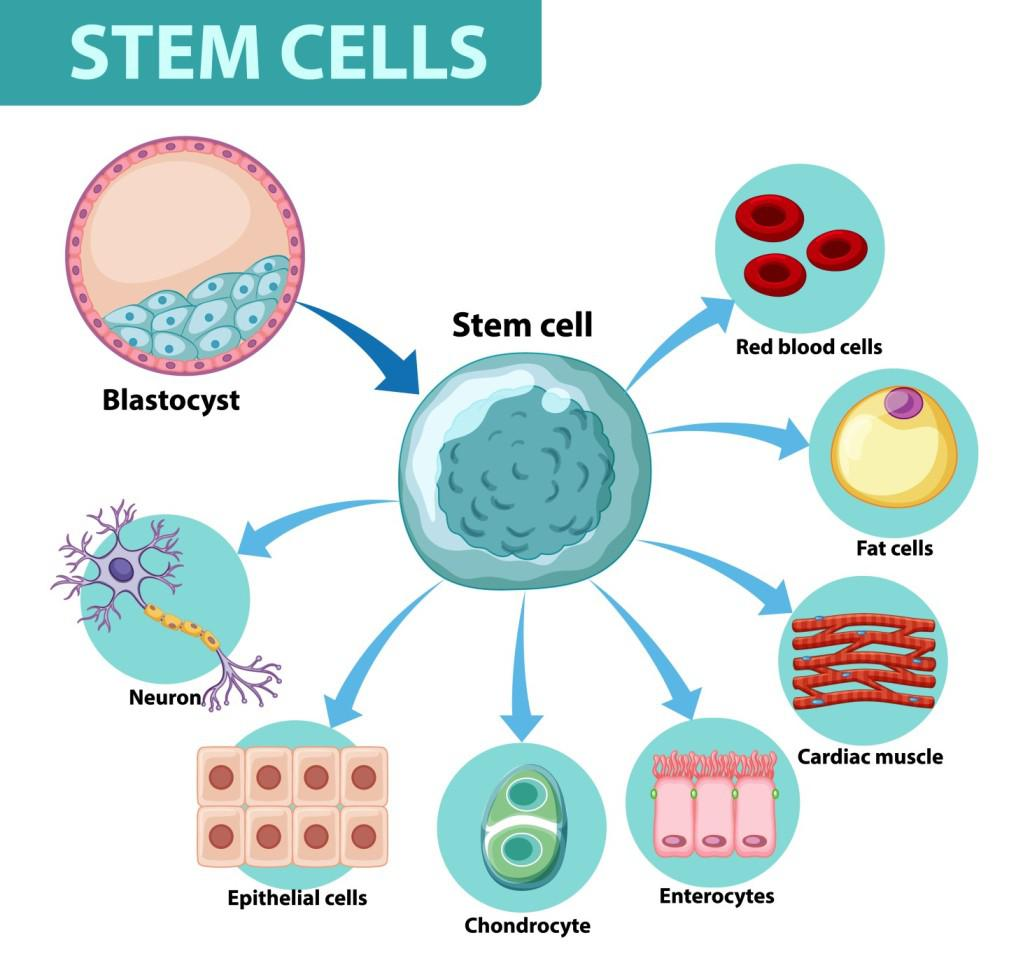
- Self-renewal — ability to divide and produce more stem cells without differentiating.
- Differentiation — ability to become specialised cells with specific functions.
- Present in both embryos (embryonic stem cells) and certain adult tissues (adult stem cells).
- Controlled by internal genetic factors and external signals from the stem cell niche.
🧠 Examiner Tip: Always link the type of potency with an example when answering application questions.
📌 Stem Cell Niches
- Specialised microenvironments that maintain stem cell properties.
- Provide chemical signals, cell-to-cell contact, and physical support.
- Examples:
- Bone marrow — hematopoietic stem cells produce blood cells.
- Hair follicles — stem cells regenerate hair.
- Intestinal crypts — stem cells replace gut lining cells every few days.
🌍 Real-World Connection: Research into recreating niches in vitro is key to improving stem cell therapies.
📌 Levels of Potency
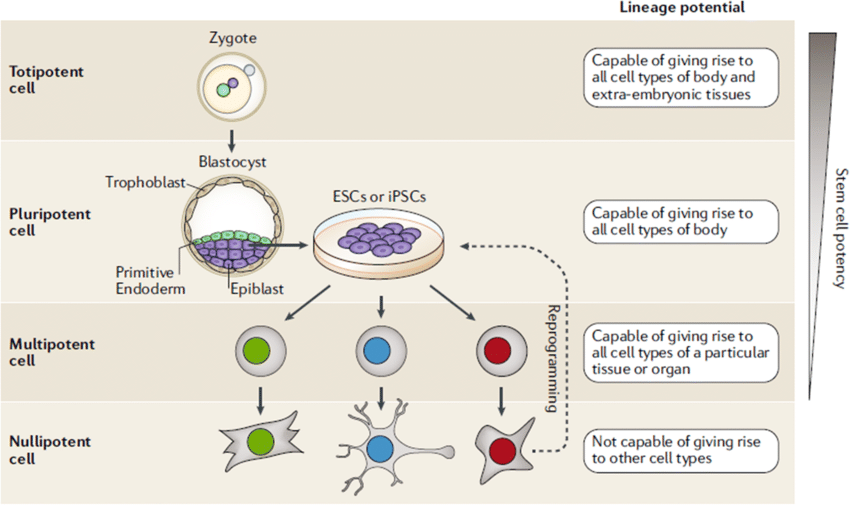
- Totipotent — zygote and early embryonic cells; can form an entire organism including placenta.
- Pluripotent — embryonic stem cells from blastocyst; can form all body cells but not placenta.
- Multipotent — adult stem cells (e.g., hematopoietic) producing a limited set of cells.
- Unipotent — satellite cells in muscle; can only produce muscle cells.
🔍 TOK Perspective: The ethics of embryonic stem cell research highlight tensions between scientific potential and moral considerations.
📌 Examples of Potency in Development
- Zygote — totipotent, capable of producing all embryonic and extraembryonic tissues.
- Inner cell mass of blastocyst — pluripotent, leading to all body tissues.
- Bone marrow — multipotent, producing red blood cells, white blood cells, platelets.
- Skin stem cells — unipotent, producing keratinocytes.