B1.1.2 – CARBOHYDRATES: STRUCTURE, FUNCTIONS & VARIETY
📌Definition Table
| Term | Definition |
|---|---|
| Monosaccharide | Simplest carbohydrate monomer (e.g., glucose, fructose, galactose). |
| Disaccharide | Carbohydrate formed when two monosaccharides join via a glycosidic bond (e.g., maltose, sucrose, lactose). |
| Polysaccharide | Large carbohydrate polymer made of many monosaccharides (e.g., starch, glycogen, cellulose). |
| Glycosidic bond | Covalent bond formed between monosaccharides in condensation reactions. |
| Isomer | Molecules with the same molecular formula but different structural arrangements (e.g., α-glucose vs β-glucose). |
| Cellulose | Structural polysaccharide made of β-glucose, found in plant cell walls. |
| Glycogen | Highly branched polysaccharide of α-glucose used for energy storage in animals. |
📌Introduction
Carbohydrates are one of the four main classes of biological macromolecules and serve as a primary energy source, storage material, and structural component in living organisms. They are composed of carbon, hydrogen, and oxygen, usually in the ratio C:H:O = 1:2:1. Their variety arises from different monomers (monosaccharides), linkages (glycosidic bonds), and structures (branched, straight, helical, or fibrous). This structural diversity underpins their multiple roles in metabolism, storage, and biological architecture.
📌 Monosaccharides
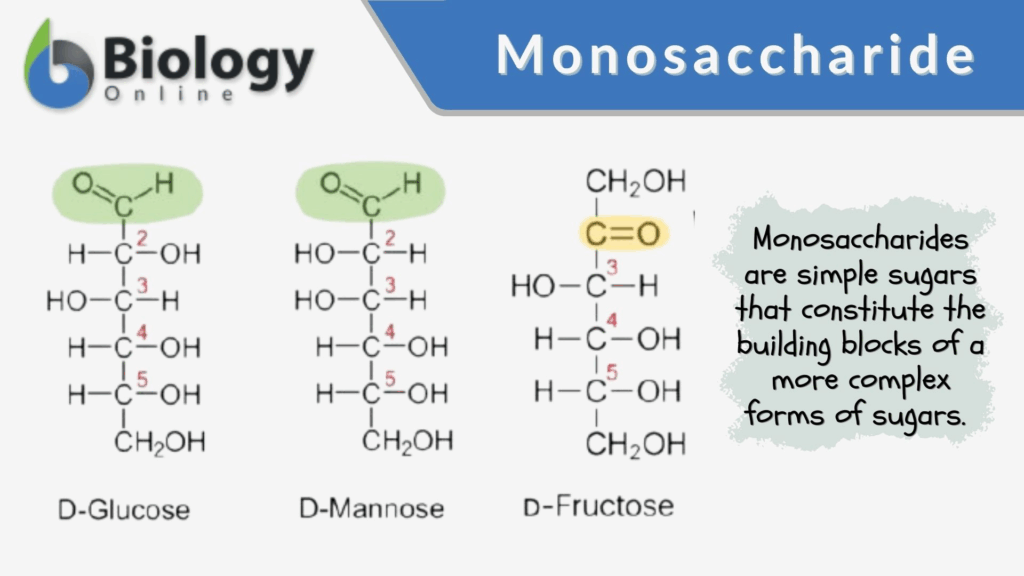
- General formula: CₙH₂ₙOₙ.
- Classified by number of carbon atoms:
- Trioses (3C) → glyceraldehyde.
- Pentoses (5C) → ribose (in RNA), deoxyribose (in DNA).
- Hexoses (6C) → glucose, galactose, fructose.
- Glucose is the most important hexose, existing in two isomers: α-glucose and β-glucose.
- Structural differences between α- and β-glucose affect the properties of their polymers.
🧠 Examiner Tip: You must be able to draw and label both α-glucose and β-glucose and identify the difference at carbon 1.
📌 Disaccharides
- Formed by condensation reactions between two monosaccharides.
- Examples:
- Maltose = glucose + glucose.
- Sucrose = glucose + fructose.
- Lactose = glucose + galactose.
- Hydrolyzed back into monosaccharides by enzymes.

🧬 IA Tips & Guidance: Food testing experiments (e.g., Benedict’s test for reducing sugars, using lactase to hydrolyze lactose) provide simple but effective investigations into carbohydrate chemistry.
📌 Polysaccharides: Storage and Structure
- Starch: α-glucose storage in plants, composed of amylose (helix, compact) and amylopectin (branched).
- Glycogen: α-glucose storage in animals, highly branched, enabling rapid glucose release.
- Cellulose: β-glucose chains, alternating monomers invert, hydrogen bonds form microfibrils → strong plant cell walls.
🌐 EE Focus: An EE could compare the digestibility of starch vs cellulose in different organisms, or investigate enzyme specificity for α- and β-linked glucose polymers.
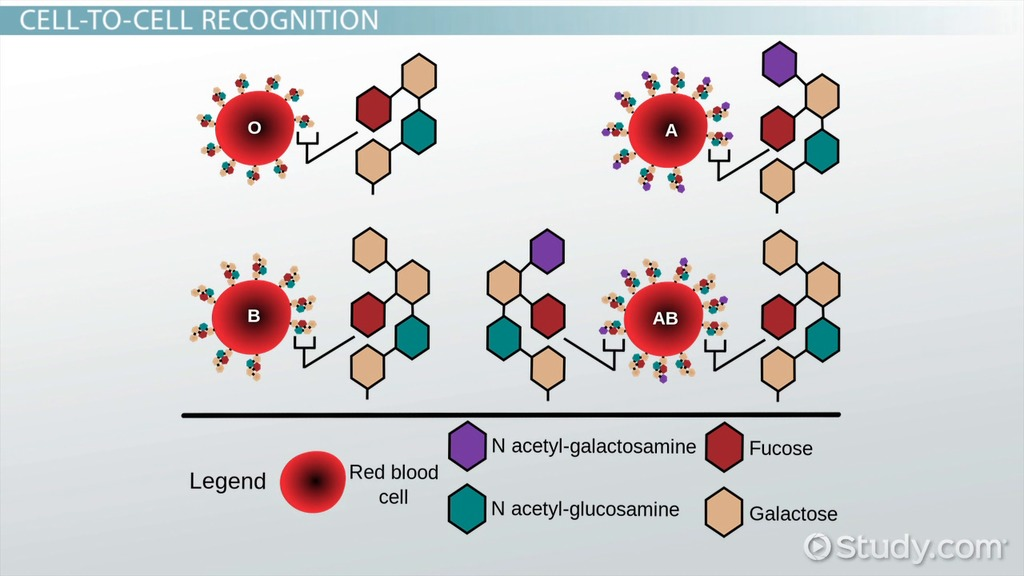
📌 Role of Glycoproteins
- Carbohydrates attached to proteins in membranes form glycoproteins.
- Functions include:
- Cell recognition and signaling.
- Immune responses (ABO blood group antigens).
- Hormone and neurotransmitter receptor activity.
❤️ CAS Link: Students could run a health awareness campaign by testing food samples for carbohydrate types and linking results to dietary energy intake.
🌍 Real-World Connection: Carbohydrates are central to health and industry. Lactose intolerance arises from inability to hydrolyze lactose. Diabetes involves impaired glucose regulation. Industrial uses include cellulose for textiles and biofuels, and starch in food processing and biodegradable plastics.
📌 Applications of Carbohydrates in Biology
- Medicine: Glycoprotein antigens determine blood type.
- Agriculture: Crop yield and storage rely on carbohydrate content.
- Biotechnology: Carbohydrate-based nanomaterials used in drug delivery.
- Food industry: Modified starches act as thickeners and stabilizers.
🔍 TOK Perspective: Carbohydrates demonstrate how small structural changes (α vs β glucose) create entirely different biological roles. TOK reflection: Does knowledge depend more on molecular detail or on functional context when classifying biological molecules?