B1.1.1 – PROPERTIES OF CARBON AND MACROMOLECULES
📌Definition Table
| Term | Definition |
|---|---|
| Carbon | Element with atomic number 6; tetravalent (forms 4 covalent bonds) allowing structural diversity in organic compounds. |
| Covalent bond | Strong bond formed when atoms share electron pairs. |
| Monomer | Small basic unit that can join with others to form polymers (e.g., glucose, amino acids). |
| Polymer | Large molecule made of repeating monomer units (e.g., starch, proteins, DNA). |
| Macromolecule | Very large biological molecule (proteins, nucleic acids, polysaccharides, lipids). |
| Condensation reaction | Reaction where covalent bonds form between monomers, releasing water. |
| Hydrolysis | Breaking of covalent bonds in polymers by adding water, releasing monomers. |
| Functional groups | Specific groups of atoms (–OH, –COOH, –NH₂, –PO₄³⁻) that determine chemical reactivity. |
📌Introduction
Carbon is the chemical foundation of all life on Earth, forming the backbone of carbohydrates, lipids, proteins, and nucleic acids. Its unique ability to form four covalent bonds allows it to build an almost limitless variety of stable yet versatile molecules, from simple chains to complex rings and branched structures. These carbon-based macromolecules are essential for storing energy, transmitting genetic information, catalyzing reactions, and building cell structures. Understanding carbon’s bonding properties and the formation of macromolecules provides the basis for molecular biology, biochemistry, and biotechnology.
📌 Chemical Properties of Carbon
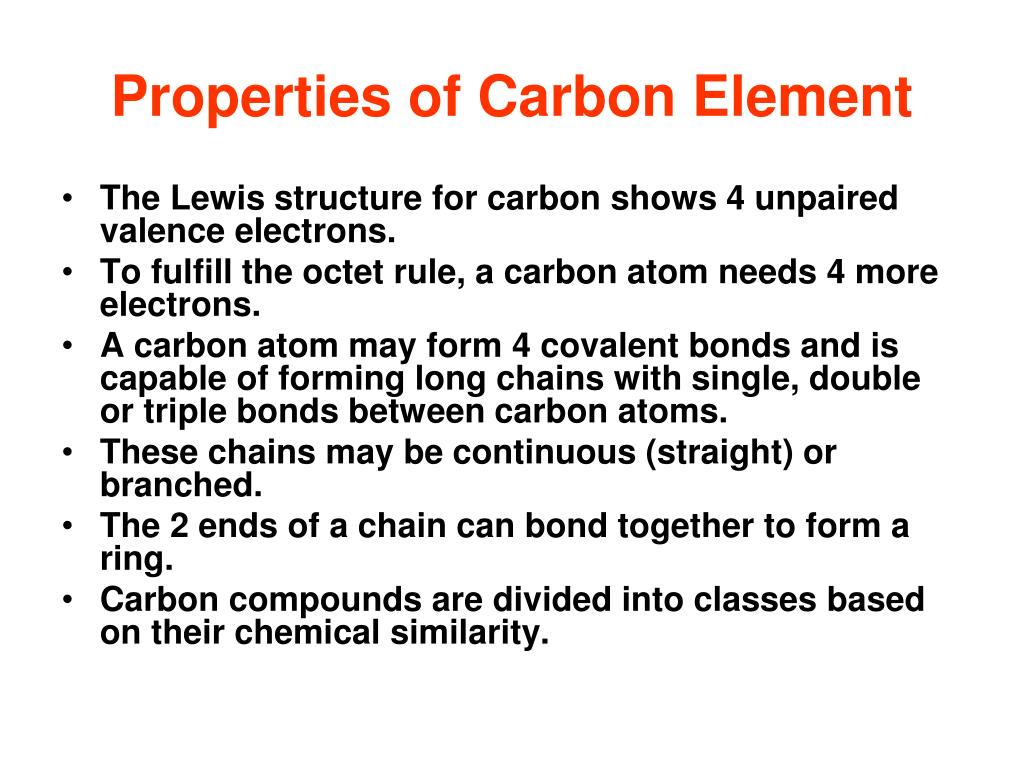
- Tetravalent: can form 4 covalent bonds → stable, complex structures.
- Can bond to itself → chains, branched molecules, and rings.
- Can form single, double, or triple bonds with C and other atoms.
- Functional groups provide specific chemical properties (e.g., hydroxyl makes molecules polar).
- Carbon compounds can adopt 3D tetrahedral shapes, influencing biological function.
🧠 Examiner Tip: Always emphasize that it is carbon’s tetravalency that makes life possible — this is a frequent IB marking point.
📌 Formation and Breakdown of Macromolecules
- Condensation Reactions:
- Monomers join to form polymers.
- Water is released.
- Examples:
- Monosaccharides → Polysaccharides (glycosidic bonds).
- Amino acids → Polypeptides (peptide bonds).
- Nucleotides → Nucleic acids (phosphodiester bonds).
- Glycerol + fatty acids → Triglycerides (ester bonds).
- Hydrolysis Reactions:
- Polymers broken into monomers.
- Water is used to break covalent bonds.
- Essential in digestion and recycling of biomolecules.
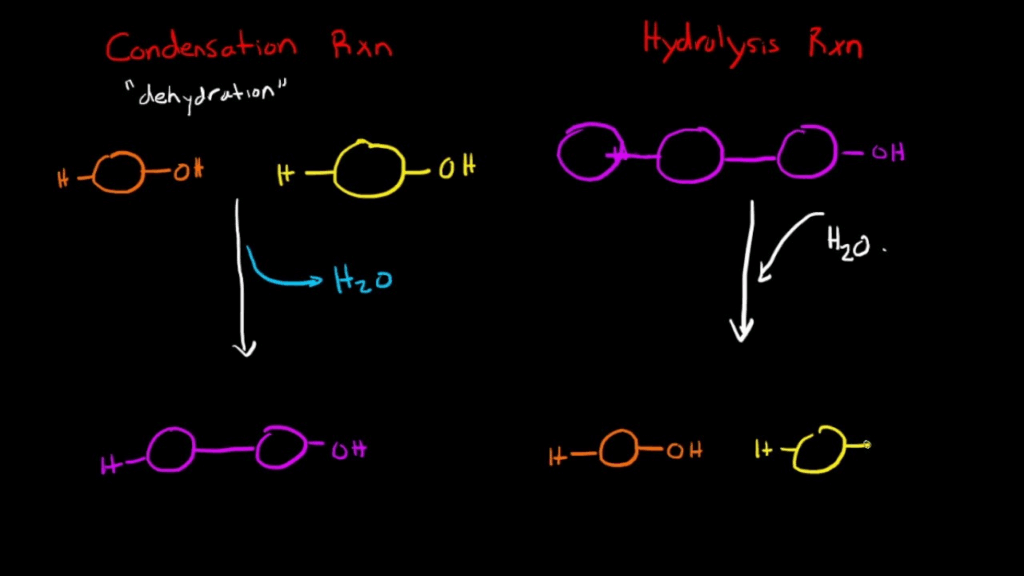
🧬 IA Tips & Guidance: Enzyme experiments (e.g., amylase breaking starch → maltose) are excellent demonstrations of hydrolysis. Ensure you connect reaction type (condensation/hydrolysis) to observed outcomes.:
📌 Major Biological Macromolecules

- Carbohydrates: Monosaccharides joined by glycosidic bonds → energy storage (starch, glycogen) and structure (cellulose).
- Proteins: Amino acids joined by peptide bonds → enzymes, hormones, structural support.
- Nucleic acids: Nucleotides joined by phosphodiester bonds → store and transmit genetic information (DNA, RNA).
- Lipids: Not true polymers; formed by glycerol + fatty acids → long-term energy storage, membranes, hormones.
🌐 EE Focus: An EE could investigate how condensation and hydrolysis reactions are catalyzed by enzymes, or how structural diversity in carbon-based molecules enables biochemical specialization.
📌 Digestion and Metabolism of Macromolecules
- Hydrolysis of polysaccharides → monosaccharides for respiration.
- Hydrolysis of polypeptides → amino acids for protein synthesis.
- Hydrolysis of triglycerides → fatty acids and glycerol for energy storage or metabolic water.
- Hydrolysis of nucleic acids → nucleotides for DNA/RNA synthesis.
❤️ CAS Link: Students can design educational models showing how food macromolecules (bread, eggs, oils) break down into their basic units, linking classroom biology to nutrition and health awareness.
🌍 Real-World Connection: Knowledge of carbon and macromolecules is central to medicine, nutrition, and biotechnology. For example, understanding hydrolysis helps in treating lactose intolerance, while polymer science underpins drug delivery systems and biodegradable plastics.
📌 Applications of Macromolecules in Biology
- Medicine: Protein-based drugs (e.g., insulin) and nucleic acid therapies (mRNA vaccines).
- Food industry: Enzymes used to hydrolyze starch into simple sugars for sweeteners.
- Agriculture: Knowledge of macromolecules aids in creating nutrient-rich animal feed.
- Biotechnology: Genetic engineering relies on manipulating nucleic acids and proteins.
🔍 TOK Perspective: Models of macromolecules (ball-and-stick, Fischer projections, space-filling diagrams) are simplifications of reality. TOK reflection: To what extent do different representations of molecules shape the way we perceive biological processes?