A2.1.1 – FORMATION OF CARBON COMPOUNDS & EARLY ORGANIC MOLECULES
📌Definition Table
| Term | Definition |
|---|---|
| Abiogenesis | The origin of life from non-living matter under early Earth conditions. |
| Organic Molecule | Carbon-containing compound, typically associated with life. |
| Monomer | Small organic molecule that can join to form polymers. |
| Polymer | Large molecule made of repeating monomer units. |
| Catalysis | Process that speeds up chemical reactions without being consumed. |
📌Introduction
The early Earth provided conditions that allowed simple carbon compounds to form spontaneously. These molecules became the building blocks for life, setting the stage for the evolution of complex biological systems.
📌 Carbon’s Bonding Properties
- Carbon forms four covalent bonds → allows complex molecules.
- Can bond with C, H, O, N, P, S to form diverse structures.
- Forms chains, branched molecules, and rings.
- Double and triple bonds add variability.
- Backbone for carbohydrates, lipids, proteins, nucleic acids.
- Versatility makes carbon unique among elements.
🧠 Examiner Tip: Always mention carbon’s tetravalency when explaining why it is suited for life.
📌 Early Earth Conditions
- Atmosphere rich in CH₄, NH₃, H₂, and H₂O vapour.
- Frequent volcanic activity and lightning.
- High UV radiation due to lack of ozone.
- Oceans acted as reaction sites (“primordial soup”).
- No oxygen — reducing environment promoted synthesis.
🧬 IA Tips & Guidance: Model chemical synthesis in a closed system under varied gas compositions.
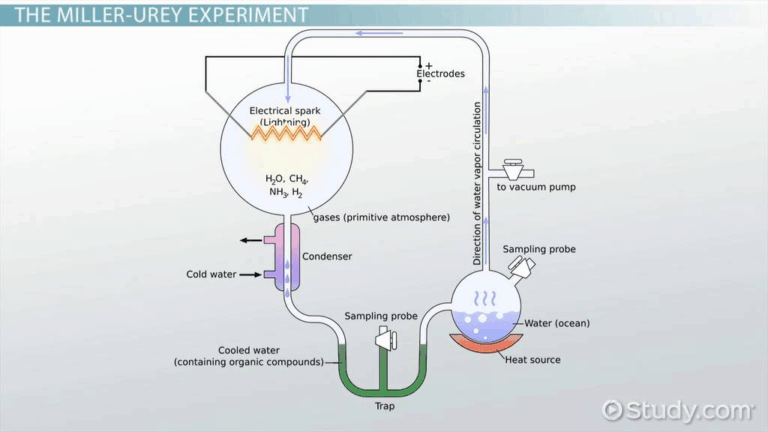
📌 Miller–Urey Experiment (1953)
- Simulated early Earth conditions in a closed apparatus.
- Gases: methane, ammonia, hydrogen, water vapour.
- Electric sparks simulated lightning.
- After a week, amino acids and other organics formed.
- Proved abiotic synthesis of organics was possible.
- Demonstrated importance of environment in chemical evolution.
🌐 EE Focus: Compare organic yields under varying atmospheric gas compositions.
📌 Formation of Monomers
- Simple molecules formed via atmospheric reactions.
- Amino acids from carbon, hydrogen, oxygen, nitrogen.
- Nucleotides from sugar, phosphate, nitrogenous base.
- Fatty acids from carbon chains.
- Monomers accumulated in oceans.
- Could be catalyzed by mineral surfaces.
❤️ CAS Link: Create a science fair model demonstrating abiotic amino acid formation.
📌 Polymerization
- Monomers joined via condensation reactions.
- Removal of water forms covalent bonds.
- Possible catalysts: clay minerals, metal ions.
- Produces proteins, nucleic acids, polysaccharides.
- Requires concentration of monomers (e.g., tidal pools).
- Step toward formation of functional macromolecules.
🌍 Real-World Connection: Similar prebiotic chemistry is studied on icy moons like Europa and Enceladus.
📌 Role of Catalysts in Early Chemistry
- Minerals and metal sulfides catalyzed polymerization.
- UV light could drive photochemical reactions.
- Hydrothermal vents supplied heat and minerals.
- Surfaces provided sites for molecular assembly.
- Catalysis increased reaction efficiency.
🔍 TOK Perspective: Scientific reconstructions of early Earth are models — their validity depends on available evidence and assumptions.