B1.1.2 – CARBOHYDRATES: STRUCTURE, FUNCTIONS & VARIETY
📌Definition Table
| Term | Definition |
|---|---|
| Monosaccharide | Single sugar unit with general formula CₙH₂ₙOₙ (e.g., glucose, ribose). |
| Disaccharide | Sugar composed of two monosaccharides joined by a glycosidic bond. |
| Polysaccharide | Large carbohydrate polymer of monosaccharide units linked by glycosidic bonds. |
| Glycosidic Bond | Covalent bond between two monosaccharides formed in a condensation reaction. |
| Isomer | Molecules with the same chemical formula but different structural arrangements. |
| Glycoprotein | Protein with carbohydrate chains attached, often involved in cell recognition. |
📌Introduction
Carbohydrates are organic molecules made of carbon, hydrogen, and oxygen in a 1:2:1 ratio. They range from small, soluble monosaccharides to large, insoluble polysaccharides. Carbohydrates serve as energy sources, structural components, and recognition molecules in cells. Variations in structure, such as α- and β-glucose, lead to diverse biological functions.
❤️ CAS Link: Conduct a nutrition awareness project teaching communities how to interpret carbohydrate content on food labels and its link to energy needs.
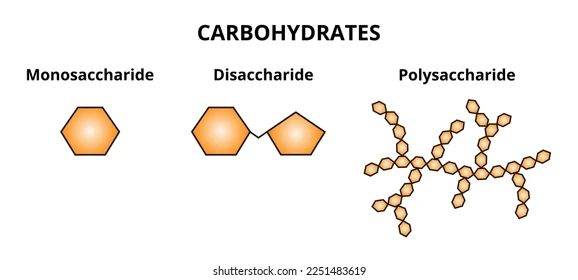
📌 Monosaccharides
- Basic building blocks of carbohydrates.
- Common examples: glucose, galactose, fructose, ribose.
- α-glucose and β-glucose differ in orientation of hydroxyl group on C1 — critical for polysaccharide structure.
- Highly soluble in water, making them easy for transport in the bloodstream or sap.
- Serve as immediate energy sources in cellular respiration.
- Ribose is a key component of RNA and ATP.
🧠 Examiner Tip: In structural questions, always draw α- and β-glucose accurately — hydroxyl group position on C1 must be correct for marks.
📌 Disaccharides
- Formed by condensation of two monosaccharides.
- Examples:
- Maltose = glucose + glucose
- Sucrose = glucose + fructose
- Lactose = glucose + galactose
- Glycosidic bonds (α-1,4 or β-1,4) determine digestibility and function.
- Soluble but less so than monosaccharides.
- Transport form of sugar in some organisms (e.g., sucrose in plants).
🌍 Real-World Connection: Lactose intolerance is caused by lack of lactase enzyme, leading to gastrointestinal symptoms when lactose remains undigested.
📌 Polysaccharides
- Long chains of monosaccharides linked by glycosidic bonds.
- Starch: Plant energy store — amylose (unbranched helix) and amylopectin (branched).
- Glycogen: Animal energy store, highly branched for rapid glucose release.
- Cellulose: Structural polysaccharide in plant cell walls; β-glucose with alternating orientation for hydrogen bonding.
- Insoluble, making them good for storage or structure.
- Different branching patterns affect digestibility and function.
🌐 EE Focus: An EE could compare enzyme activity on α-linked vs β-linked polysaccharides, exploring implications for digestibility.
📌 Glycoproteins and Recognition
- Carbohydrate chains attached to proteins on cell membranes.
- Play roles in cell recognition, immune responses, and signalling.
- Determine blood types (A, B, AB, O) through surface antigens.
- Pathogens may mimic host glycoproteins to evade immunity.
- Essential in cell–cell adhesion and communication.
- Often involved in receptor–ligand interactions.
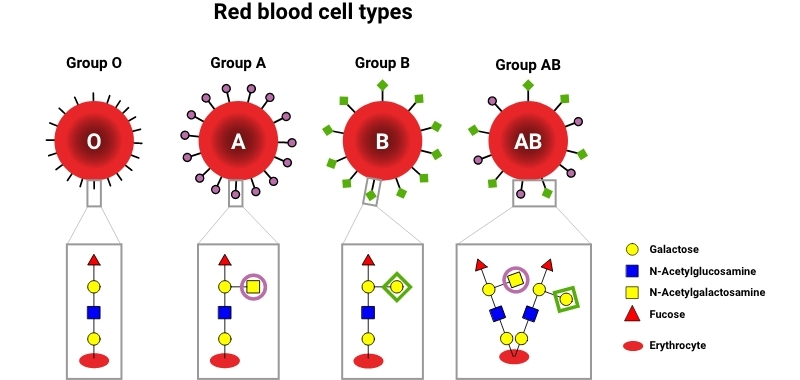
🔍 TOK Perspective: How does our classification of carbohydrates by structure reflect human-created categories rather than natural boundaries?