B1.1.1 – PROPERTIES OF CARBON AND MACROMOLECULES
📌Definition Table
| Term | Definition |
|---|---|
| Carbon Skeleton | The chain or ring of carbon atoms forming the structural backbone of an organic molecule. |
| Functional Group | A specific group of atoms that imparts characteristic chemical properties to a molecule. |
| Monomer | Small, repeating unit that can be joined to form polymers. |
| Polymer | Large molecule made of repeating monomer units. |
| Condensation Reaction | Chemical reaction where monomers join, releasing water. |
| Hydrolysis Reaction | Chemical process that breaks polymers into monomers using water. |
📌Introduction
Carbon’s ability to form four covalent bonds and create diverse structures underpins all biological macromolecules. The arrangement of carbon atoms — in chains, rings, and branched frameworks — allows the formation of an immense variety of molecules, from small sugars to large proteins. The process of linking smaller subunits into large macromolecules involves condensation reactions, while breakdown involves hydrolysis.
❤️ CAS Link: Organise a hands-on molecular modelling workshop for younger students, using kits to build carbon-based macromolecules and demonstrate condensation vs. hydrolysis.
📌 Properties of Carbon
- Carbon forms four covalent bonds (tetravalency) allowing stable and diverse molecules.
- Can form single, double, and triple bonds with itself or other atoms.
- Structures include straight chains, branched chains, and rings.
- Carbon-carbon bonds are strong and stable, allowing large complex molecules.
- Functional groups (e.g., hydroxyl, carboxyl, amino) attached to the carbon skeleton influence molecular properties.
- This versatility is the basis for the diversity of life’s molecules.
🧠 Examiner Tip: When explaining carbon’s importance, always mention both bonding capacity and structural diversity for maximum marks.
📌 Monomers, Polymers, and Macromolecules
- Monomers are small units like monosaccharides, amino acids, nucleotides, and fatty acids.
- Polymers are long chains of monomers linked by covalent bonds (e.g., starch, proteins, DNA).
- Macromolecules are large biological molecules that may be polymers (carbohydrates, proteins, nucleic acids) or non-polymers (lipids).
- Polymerisation occurs via condensation reactions (water released).
- Breakdown occurs via hydrolysis (water added).
- The type and sequence of monomers determine the function of the macromolecule.
🌍 Real-World Connection: The condensation reaction between glucose molecules to form starch is exploited in food manufacturing for texture and storage stability.
📌 Condensation and Hydrolysis Reactions
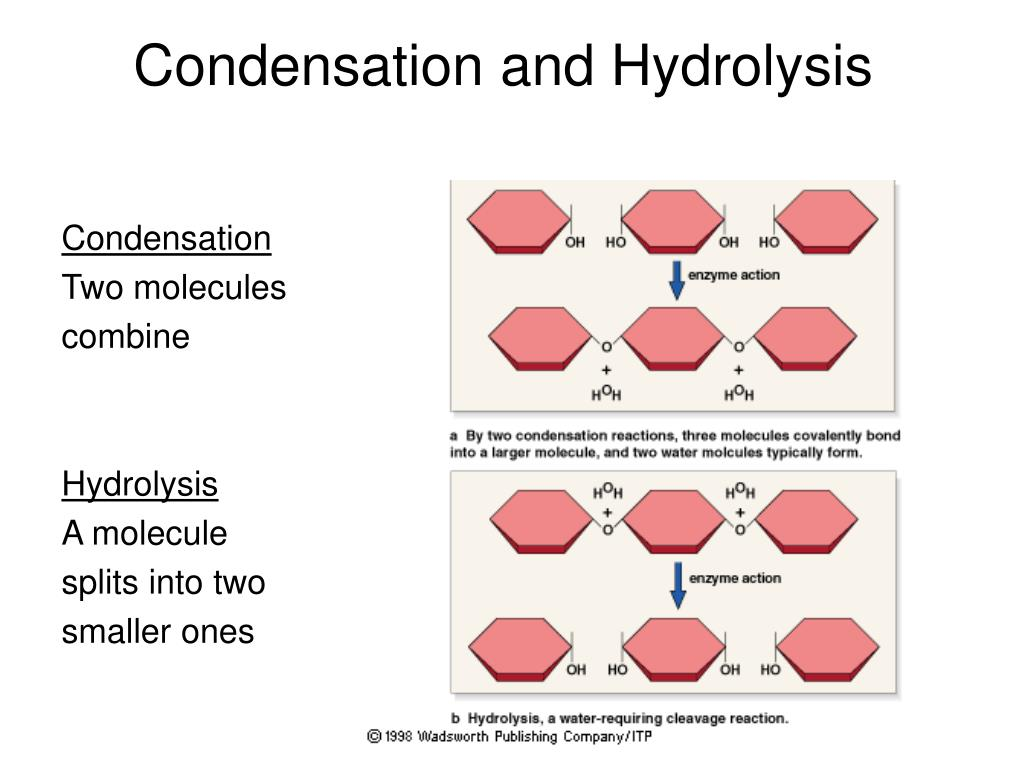
Condensation: Joins monomers → polymer + water; catalysed by specific enzymes.
Hydrolysis: Breaks polymers → monomers by adding water; catalysed by hydrolase enzymes.
Reactions are reversible under appropriate conditions.
Examples:
- Glucose + glucose → maltose + water (condensation).
- Maltose + water → glucose + glucose (hydrolysis).
Both reactions are crucial in metabolism (anabolism vs. catabolism).
Energy changes: condensation often requires energy input; hydrolysis releases energy.
🌐 EE Focus: An EE could investigate enzyme specificity in hydrolysis reactions, comparing activity of different carbohydrases or proteases.