8.3 URBAN AIR POLLUTION
📌 Definitions Table
| Term | Definition (Exam-Ready, 2 Marks) |
|---|---|
| Urban Smog | Air pollution in cities caused by the accumulation of industrial and vehicular emissions, often worsened by temperature inversions. |
| Pedestrianising | The process of converting streets or areas for pedestrian-only use to reduce traffic, pollution, and improve urban livability. |
| Green Walls | Vertical structures covered with vegetation that reduce heat, filter air pollutants, and enhance urban biodiversity. |
| Scrubbers | Pollution control devices used in industrial settings to remove harmful gases and particulates from exhaust emissions. |
- 🧠 Exam Tips:
For urban air pollution terms, always mention sources and impacts on health or climate when elaborating.
Connect green walls and pedestrianising to urban sustainability or heat island mitigation in evaluation questions.
📌 Causes of Urban Air Pollution
- Human activities that release harmful substances into the atmosphere cause urban air pollution
- Pollutants in the air can come from many sources and impact both human health and the environment
- Common pollutants include:
- Nitrogen oxides (NOx)
- Sulphur dioxide (SO2)
- Carbon monoxide (CO)
- Particulate matter (PM)
- Particulate matter refers to tiny solid particles or liquid droplets in the air
- These particles can come from dust, soot, smoke, and vehicle emissions
- Particulate matter can be classified by size:
- PM2.5: fine particles with a diameter of 2.5 micrometres or smaller
- PM10: larger particles with a diameter of 10 micrometres or smaller
🌐 EE Tip: Study air pollution or heat island effects in different parts of your city, linking to socio-economic variables.
Primary pollutants
- Primary pollutants are harmful substances that are:
- Directly emitted from a source
- Immediately active in the atmosphere
- They enter the air through various activities like burning fossil fuels, industrial processes, or natural events such as volcanic eruptions
Sources of primary pollutants
- Natural sources:
- Some air pollutants come from natural events that occur without human involvement
- Forest fires: release smoke, ash, and particulate matter into the air
- Dust storms: strong winds lift dust from dry areas, which spreads to cities
- Volcanic eruptions: these produce large amounts of SO2 and ash
- Some air pollutants come from natural events that occur without human involvement
- Anthropogenic (human-made) sources:
- Many pollutants in urban areas come from human activities, especially those involving the burning of fuels
- Burning fossil fuels: emissions from vehicles, power plants, and factories produce NOx, SO2, CO, and PM
- Agricultural burning and deforestation: these release large quantities of smoke, dust, and other pollutants into the atmosphere
- Construction sites and roads: create dust and PM from the movement of machinery and vehicles
- Industrial processes: factories release pollutants like NOx and PM from smokestacks and chemical processing
- Many pollutants in urban areas come from human activities, especially those involving the burning of fuels
Common pollutants from urban activities
- The most common pollutants in urban areas are usually linked to the combustion of fossil fuels
- Particulate matter (PM2.5 and PM10): tiny particles from exhaust fumes, industrial activities, and construction dust
- CO: released by cars and industrial processes that burn fuels
- NOx: produced by vehicle emissions and power plants
- SO2: released mainly by burning coal and oil
Secondary pollutants
- Secondary pollutants are not emitted directly but form in the atmosphere when primary pollutants react with other chemicals
- Tropospheric ozone (O3): forms when nitrogen oxides (NOx) react with sunlight
- It is a major component of urban smog
- Tropospheric ozone (O3): forms when nitrogen oxides (NOx) react with sunlight
Examples of urban air pollution
- Beijing, China: often experiences high levels of PM2.5, mainly due to coal burning for energy and industrial activity
- Los Angeles, USA: struggles with ozone pollution due to a high number of vehicles and sunny weather, which speeds up the reaction that forms ozone
- The burning of crops, industrial activity, and vehicle emissions frequently cause severe air pollution inNew Delhi, India
📌 Air Pollution Management Strategies
- Air pollution management strategies are designed to reduce harmful emissions and improve air quality in urban areas
- These strategies focus on:
- Reducing the sources of pollution
- Promoting cleaner technologies
- Encouraging sustainable urban living
Reducing the use of fossil fuels
- One of the most effective ways to manage urban air pollution is to reduce the reliance on fossil fuels
- This includes:
- Promoting the use of renewable energy sources like wind, solar, and hydro to power cities
- Improving public transport systems in cities to reduce car usage, e.g.
- Electric buses
- Efficient metro systems
- Creating infrastructure for cycling, e.g.
- More cycle lanes
- Cycle-hire schemes
- Pedestrianising city centres
Emission zones and car restrictions
- Emission zones are areas where only vehicles meeting certain environmental standards are allowed to enter
- Low Emission Zones (LEZs) restrict high-polluting vehicles, reducing air pollution in the city centre
- For example, London has an Ultra Low Emission Zone (ULEZ) where only vehicles meeting strict emission standards can drive
- Some cities also restrict car use on certain days or at peak times to decrease congestion and emissions
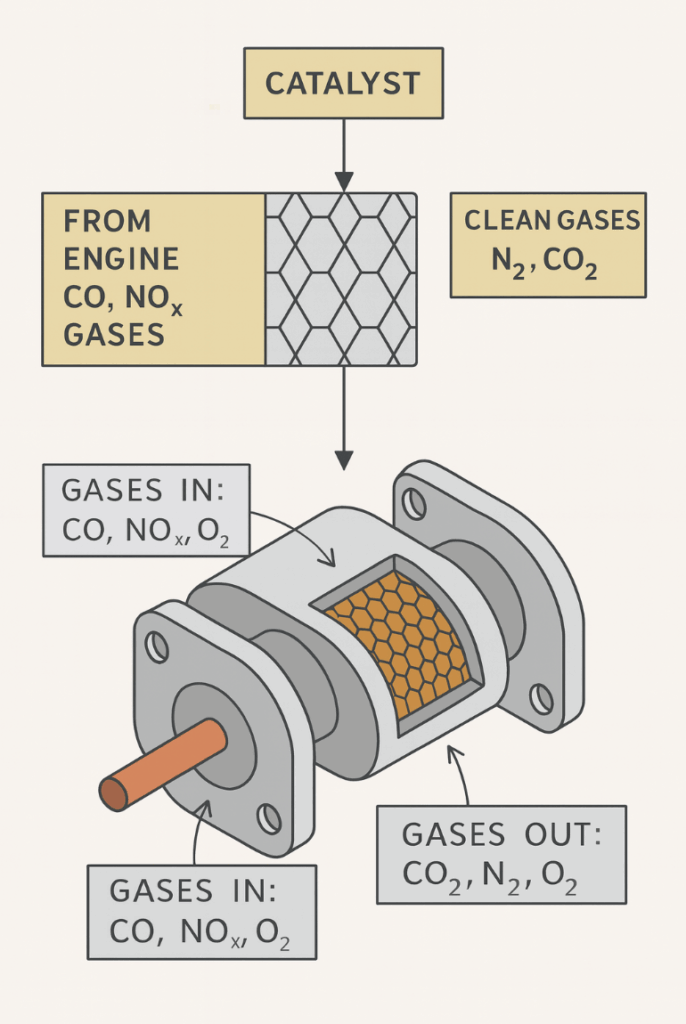
Catalytic converters
- Catalytic converters are devices fitted to car exhaust systems that reduce harmful emissions
- They contain catalysts that speed up chemical reactions to convert pollutants like nitrogen oxides and carbon monoxide into less harmful gases such as nitrogen and carbon dioxide
- In many countries, it is compulsory for vehicles to have catalytic converters
Growing trees and natural screens
- Trees and green spaces play an important role in filtering pollutants from the air
- Trees can reduce air pollution and improve air quality by:
- Absorbing carbon dioxide
- Trapping particulate matter
- Natural screens such as hedges, tree lines and green walls can also help reduce pollutants near roads and buildings
Green walls and green roofs
- Green walls and green roofs are covered with vegetation and can improve air quality by filtering pollutants
- They also help regulate temperature, reducing the urban heat island effect
📌 Acid Rain
Acid rain formation
- Acid rain refers rainfall that has a pH lower than normal rainwater
- Regular rain has a pH between 5 and 5.5, meaning it is naturally slightly acidic
- Acid rain is more acidic, has a pH lower than 5, and is frequently the result of human activity
Chemical reactions leading to acid rain
- Nitrogen oxides (NOx) and sulphur dioxide (SO2) are the main gases responsible for acid rain
- These gases react with water and oxygen in the atmosphere to form nitric acid and sulfuric acid
Formation of nitric acid
- Nitrogen oxides are mainly produced from vehicle exhausts
- The reactions are as follows:
- Nitrogen monoxide (NO) reacts with oxygen (O2) to form nitrogen dioxide (NO2)
2NO + O2 → 2NO2
- The nitrogen dioxide then reacts with water (H2O) and oxygen in the air to produce nitric acid (HNO3)
4NO2 + O2 + 2H2O → 4HNO3
Formation of sulphuric acid
- Sulphur dioxide is produced by burning fossil fuels and reacts with water in the atmosphere
- The reactions are as follows:
- Sulphur dioxide (SO2) dissolves in rainwater, producing sulphurous acid (H2SO3)
SO2 + H2O → H2SO3
- The sulphurous acid is then oxidised by oxygen in the air to produce sulfuric acid (H2SO4)
2H2SO3 + O2 → 2H2SO4
![]() Types of deposition
Types of deposition
- Wet deposition refers to acidic precipitation falling to Earth in the form of rain, snow, or fog
- Sulphuric acid and nitric acid can also combine with ash and other particles present in the air, forming dry particles (i.e. acidic dust and gases)
- Dry deposition occurs when these particles settle on surfaces, including vegetation, buildings, cars and soil
Acid rain impacts
Impacts on terrestrial habitats
- Acidic deposition from acid rain accelerates the leaching of essential nutrients from soil, such as calcium, magnesium and potassium
- Leaching of these nutrients reduces their availability for plants
- This leads to nutrient deficiencies
- This reduces plant growth and overall ecosystem productivity
- Acidic rain can increase soil toxicity
- This can occur by mobilising harmful metals like aluminium
- This damages plant roots and affects their ability to absorb water and nutrients
- Acid rain causes direct damage to foliage
- This weakens trees, making them more vulnerable to disease and harsh weather
- Coniferous forests, e.g. forests of pine or spruce trees, are sensitive to acid rain
- This is due to their shallow root systems and thin bark
- Acid rain also damages their foliage and inhibits nutrient absorption
Impacts on freshwater habitats
- Acid rain can make water bodies more acidic
- This is due to a process referred to as solubilisation of aluminium
- Acid rain causes aluminium, which is normally bound in the soil, to dissolve
- This allows the aluminium to enter nearby water bodies
- This aluminium is toxic to aquatic life, such as fish and freshwater invertebrates
- Fish gills can become coated with aluminium
- This makes it harder for them to breathe
- Some invertebrates with exoskeletons may have difficulty maintaining their protective shells
- They rely on calcium to build and maintain their hard outer shells
- When acid rain increases the acidity of water, it reduces the availability of calcium and other minerals that these organisms need
- This makes it harder for them to properly develop or maintain their exoskeletons
- Fish gills can become coated with aluminium
Impacts on buildings and infrastructure
Corrosion of construction materials
- Acid rain erodes materials like marble, limestone, steel, and paint used in buildings and monuments
- Marble and limestone both contain calcium carbonate (CaCO3)
- The calcium carbonate reacts with sulphuric acid or nitric acid, causing stonework to corrode and weaken
- For example, the Taj Mahal in India, made of marble, has shown signs of erosion and discolouration due to acid rain
- Acid rain has also had an impact on historical statues and structures, such as those in Rome and Greece
Impacts on human health
Respiratory issues
- Acid rain does not directly harm humans
- However, nitrate and sulphate particles from acid rain can cause respiratory problems
- PM2.5 particles (tiny air pollutants) from acid rain can enter the lungs
- This leads to:
- Tissue damage
- Lung inflammation
- An increased risk of conditions such as asthma and bronchitis
- As a result, areas with heavy industrial activity, such as parts of China and Eastern Europe, experience greater respiratory health risks
Acid rain management strategies
- There are three main levels of pollution management strategies:
- Changing human activity
- Regulating and reducing quantities of pollutants released at the point of emission
- Cleaning up the pollutants and restoring the ecosystem after pollution has occurred

- These levels can also be applied to acid rain management strategies
- Acid rain requires effective pollution management strategies to mitigate its harmful effects on the environment and human health
1. Altering human activity
- Reducing the consumption of fossil fuels is a key strategy to minimise acid rain
- Encourage the use of alternative energy sources, such as renewable energy, can significantly reduce emissions of sulphur dioxide and nitrogen oxides
- International agreements and national governments play a vital role in:
- Promoting sustainable practices
- Supporting the development of clean technologies
- Lobbying for emissions reductions
2. Regulating and monitoring pollutant release
- Government regulations and monitoring systems are essential to control and reduce the release of pollutants that contribute to acid rain
- Coal-burning power plants and vehicles are major sources of sulphur dioxide and nitrogen oxide emissions
- Installing pollution control devices such as scrubbers and catalytic converters can effectively remove these pollutants from emissions
3. Clean-up and restoration measures
- In areas heavily affected by acid rain, certain strategies may be used to mitigate the damage caused
- For example, spreading ground limestone or lime in acidified lakes and rivers can neutralise acidity and restore the water’s pH balance
- Restoring damaged ecosystems can also be achieved through re-colonisation efforts, such as planting acid-tolerant vegetation
- This can help restore ecological balance to these damaged ecosystems