A1.1.1 – HYDROGEN BONDING AND THE BIOLOGICAL IMPORTANCE OF WATER
📌Definition Table
| Term | Definition |
|---|---|
| Hydrogen Bond | A weak electrostatic attraction between a δ⁺ hydrogen atom and a δ⁻ atom (like oxygen or nitrogen) in another molecule. |
| Polarity | A condition where electrons are unevenly shared in a bond, resulting in partial charges. |
| Dipole | A molecule that has regions of positive and negative charge. |
| Cohesion | The attraction between molecules of the same substance (e.g., water to water). |
| Adhesion | The attraction between water molecules and other polar/charged surfaces. |
| Solvent | A liquid that dissolves solutes to form a solution; water is the universal solvent. |
📌Introduction
Water is the most essential molecule for life. It comprises 70–95% of cellular mass and acts as the medium for biochemical reactions, a solvent, a thermal regulator, and a structural support for living organisms. These biological roles are due to water’s polarity and ability to form hydrogen bonds.
📌 Structure and Polarity of Water
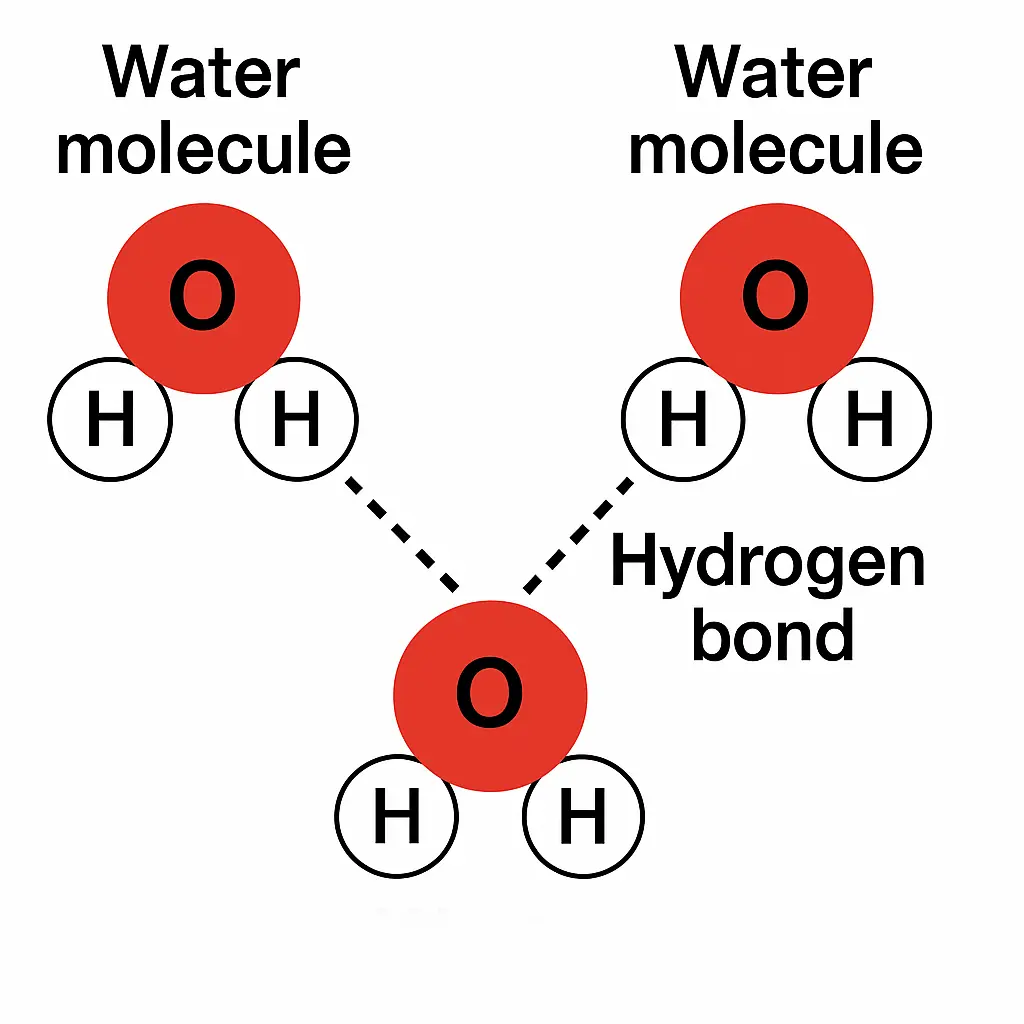
- A water molecule consists of two hydrogen atoms and one oxygen atom, forming a bent shape.
- The oxygen atom attracts electrons more strongly than hydrogen, causing uneven electron sharing.
- This creates a partial negative charge (δ⁻) on oxygen and a partial positive charge (δ⁺) on hydrogen.
- Because of this, water is a polar molecule and behaves as a dipole.
- Water molecules are attracted to each other via hydrogen bonds between opposite charges.
- These bonds are responsible for many unique properties of water like surface tension and high boiling point.
🧠 Examiner Tip: Use terms like “dipole”, “partial charges (δ⁻ / δ⁺)”, and “hydrogen bonding” when describing water’s structure or behavior. Diagrams must clearly show the orientation and labeling of water molecules and hydrogen bonds.
📌 Hydrogen Bonds in Biological Molecules
- Hydrogen bonds in DNA base pairs (A-T and G-C) stabilize the double helix structure.
- Proteins rely on hydrogen bonds to maintain secondary structures like α-helices and β-pleated sheets.
- Tertiary protein structure is also supported by hydrogen bonding between R-groups.
- In cellulose and collagen, hydrogen bonds provide structural strength and rigidity.
- Enzyme-substrate interactions are stabilized by hydrogen bonds, aiding in catalytic activity.
- Hydrogen bonding is essential in forming and maintaining biologically active molecular structures.
🧬 IA Tips & Guidance: Explore experiments involving protein denaturation, enzyme kinetics, or DNA interactions. Discuss how temperature or pH disrupts hydrogen bonding, and use molecular diagrams in your analysis to show how structure affects function.
📌 Water as a Solvent and Medium for Life
- Water’s polarity allows it to dissolve a wide variety of solutes, making it a universal solvent.
- It supports transport of nutrients and waste in the bloodstream and plant xylem/phloem.
- Water enables biochemical reactions to occur in aqueous environments inside cells.
- Polar molecules like glucose and ions dissolve easily, aiding metabolic function.
- Non-polar molecules like fats are excluded, which helps organize biological structures like membranes.
- Solubility differences affect how substances are transported or stored in organisms.
🌐 EE Focus: A strong EE topic could explore solubility of biological molecules in water, the role of polarity in reaction rates, or hydrogen bonding in protein folding. Focus your research on how molecular structure affects function in biological system
📌 Cohesion and Adhesion in Transport
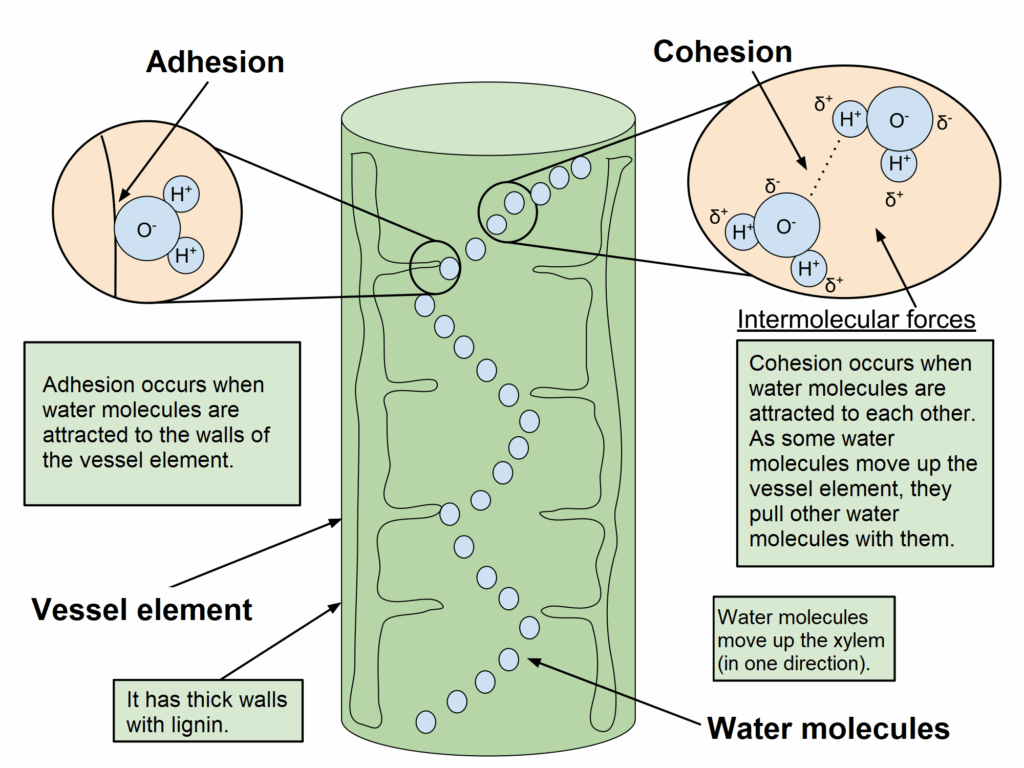
- Cohesion results from hydrogen bonds between water molecules, allowing them to stick together.
- This creates continuous water columns in plant xylem, aiding upward movement against gravity.
- Adhesion allows water to cling to xylem walls (cellulose), supporting capillary action.
- Together, cohesion and adhesion support transpiration pull and efficient water transport.
- Surface tension from cohesion enables certain insects to move across water surfaces.
- These properties are crucial for plant survival and maintaining water cycles in ecosystems.
❤️ CAS Link: Design a service project where students build simple capillary tube demonstrations or a hydroponic system. Use it to explain cohesion and adhesion while promoting sustainable agriculture and plant care awareness.
📌 Hydrogen Bonding and Thermal Stability
- Water has a high specific heat capacity due to the energy needed to break hydrogen bonds.
- It can absorb large amounts of heat with minimal temperature change.
- This property buffers temperature changes in organisms and environments.
- It maintains stable aquatic ecosystems, which support biodiversity.
- Helps organisms regulate internal temperatures and sustain enzyme activity.
- Water’s high latent heat of vaporization makes sweating an effective cooling mechanism.
🌍 Real-World Connection: Water’s heat buffering capacity is crucial in climate regulation. Global warming reduces this balance, impacting marine life, ice caps, and weather systems. Understanding hydrogen bonding helps explain these environmental shifts
📌 Water’s Role in the Origin and Continuation of Life
- Life is believed to have originated in aqueous environments, such as near hydrothermal vents.
- Water dissolved molecules and allowed chemical reactions inside primitive membranes.
- It continues to support life as the solvent of metabolism and transport medium.
- Water is required for photosynthesis, respiration, and digestion.
- It is actively involved in hydrolysis and condensation reactions.
- Scientists search for water on other planets as a key indicator of life’s potential.
🔍 TOK Perspective: Can the presence of water alone be considered sufficient evidence of life? Reflect on how evidence, assumptions, and methodology influence scientific conclusions when interpreting data from exoplanets.