D2.3.1 OSMOSIS AND SOLUTE POTENTIAL
📌Definition Table
| Term | Definition |
|---|---|
| Osmosis | Passive movement of water molecules across a selectively permeable membrane from higher water potential to lower water potential. |
| Water potential (Ψ) | Measure of the potential energy of water per unit volume; determines direction of water movement. |
| Solute potential (Ψs) | Effect of solute concentration on water potential; always negative relative to pure water. |
| Hypertonic | Solution with lower water potential than the cell, causing water to leave and cell to shrink. |
| Hypotonic | Solution with higher water potential than the cell, causing water to enter and cell to swell. |
| Isotonic | Solution with equal water potential, no net water movement. |
📌Introduction
Osmosis is fundamental to maintaining plant cell homeostasis and driving water uptake from soil. The water potential equation Ψ = Ψs + Ψp explains how solute potential and pressure potential together regulate osmosis. Solute potential decreases water potential, while pressure potential counteracts it by exerting force on the cell wall. Understanding osmosis underpins all water transport processes in plants, from root absorption to transpiration streams in leaves
📌 Principles of Osmosis
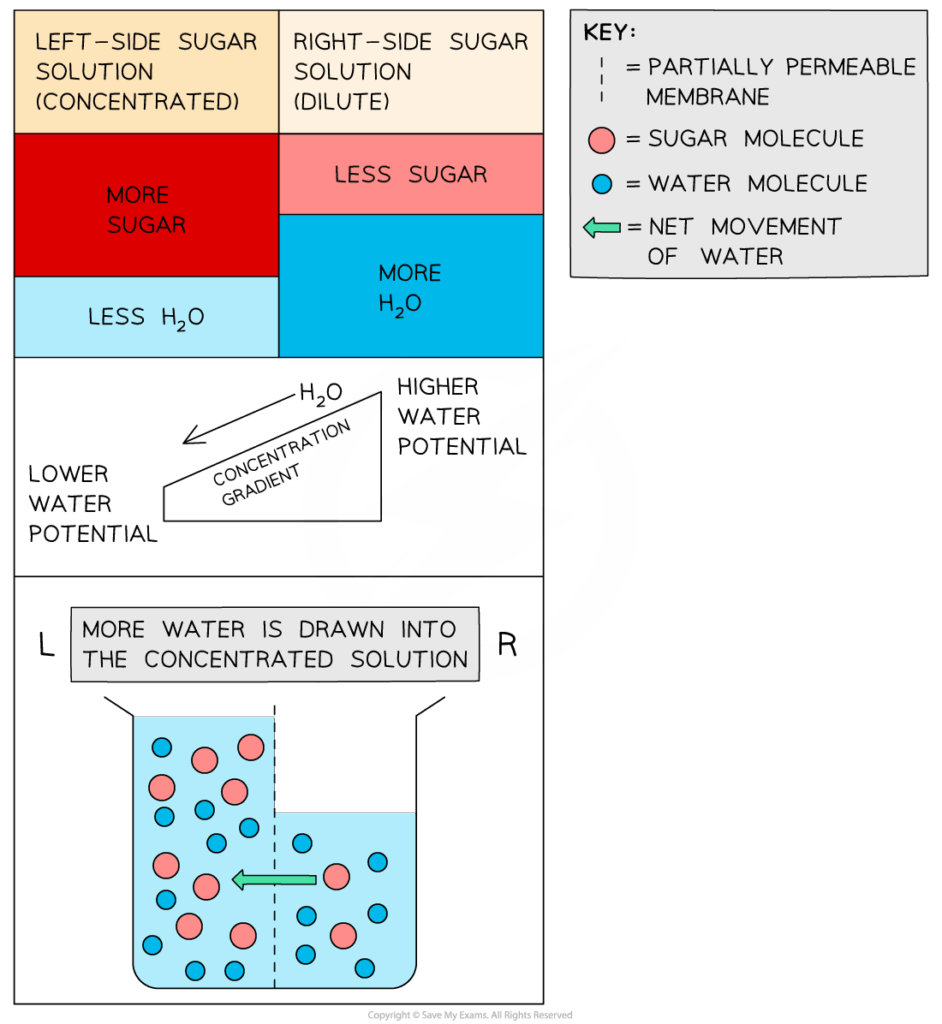
- Water moves down its potential gradient — from regions of higher Ψ to lower Ψ.
- Solute concentration decreases Ψ, making solutions more negative.
- Plant cell membranes are selectively permeable, allowing water but not large solutes.
- Cells in hypertonic solutions lose water and plasmolyse; in hypotonic, they gain water and become turgid.
- Osmosis is critical for nutrient uptake and maintaining cell shape.
🧠 Examiner Tip: Don’t confuse osmosis with diffusion. Diffusion involves solute particles, whereas osmosis is strictly about water movement across membranes.
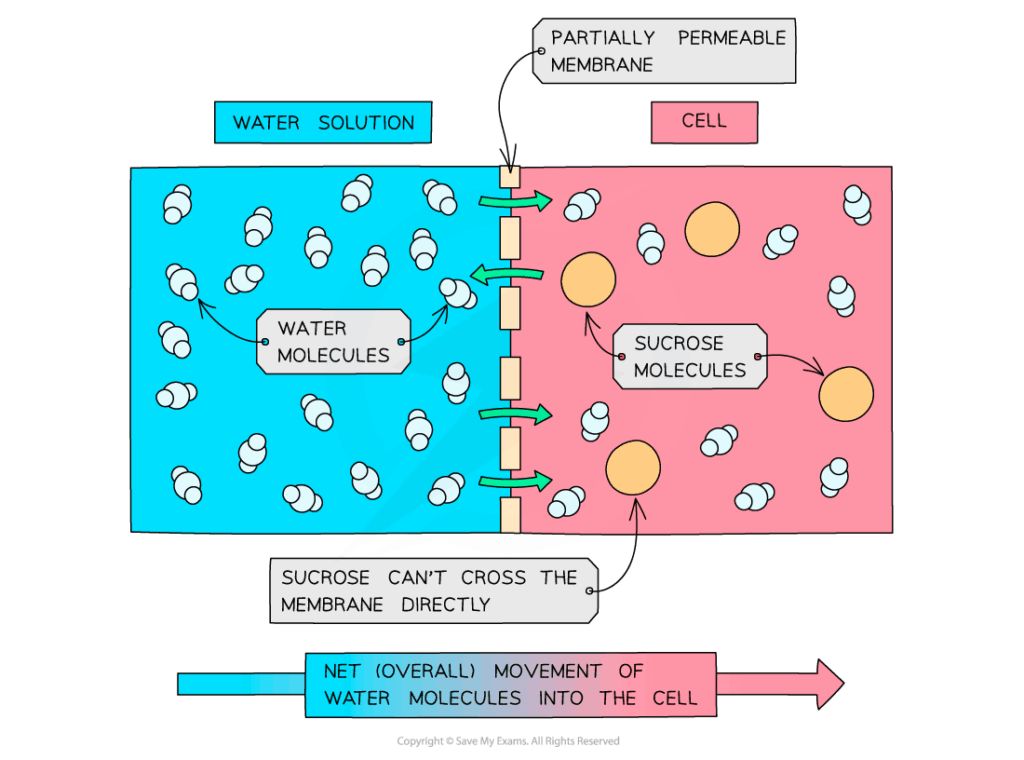
📌 Solute Potential (Ψs)
- Calculated using: Ψs = -iCRT (ionisation constant × concentration × gas constant × temperature).
- Always negative since solutes lower free water concentration.
- Higher solute concentrations → more negative Ψs → stronger pull for water.
- Explains why fertiliser salts or seawater can dehydrate plants.
- Experimental measurement involves observing plasmolysis in tissues (e.g., onion cells).
🧬 IA Tips & Guidance: Design an experiment using plant tissue (potato, onion) in different sucrose solutions to measure Ψs. Graph percentage mass change vs. solute concentration to estimate water potential.
📌 Cellular Responses to Osmosis
- Plasmolysis: cytoplasm shrinks away from cell wall in hypertonic solution.
- Turgidity: cell swells in hypotonic solution but wall prevents bursting.
- Incipient plasmolysis: point at which plasma membrane just detaches from wall; used to estimate Ψs.
- Osmosis regulates guard cell turgor → stomatal opening/closing.
- Critical in seed germination, where water uptake reactivates metabolism.
🌐 EE Focus: An EE could explore how osmotic stress (e.g., salinity) influences Ψs and plant survival, connecting to ecological distribution of species.
📌 Applications of Osmosis
- Agricultural irrigation must balance soil salinity to prevent water loss from roots.
- Food preservation uses hypertonic solutions (salt, sugar) to inhibit microbes.
- Osmotic gradients exploited in desalination and biotechnological water purification.
- Medical relevance: IV fluids must be isotonic to avoid damaging blood cells.
- Environmental stress tolerance in plants often linked to osmotic adjustments.
❤️ CAS Link: Students could demonstrate osmosis with simple potato experiments in school/community, showing real-life effects of solute concentration on water uptake.
🌍 Real-World Connection: Drought and soil salinity crises highlight the importance of understanding osmotic regulation in crops, linking plant physiology to food security.
📌 Integration into Plant Systems
- Osmosis drives initial root water uptake before bulk flow takes over.
- Interacts with pressure potential in cells to generate turgor.
- Works with transpiration pull to maintain continuous water column.
- Explains wilt recovery when water is restored.
- Foundation for understanding water transport models.
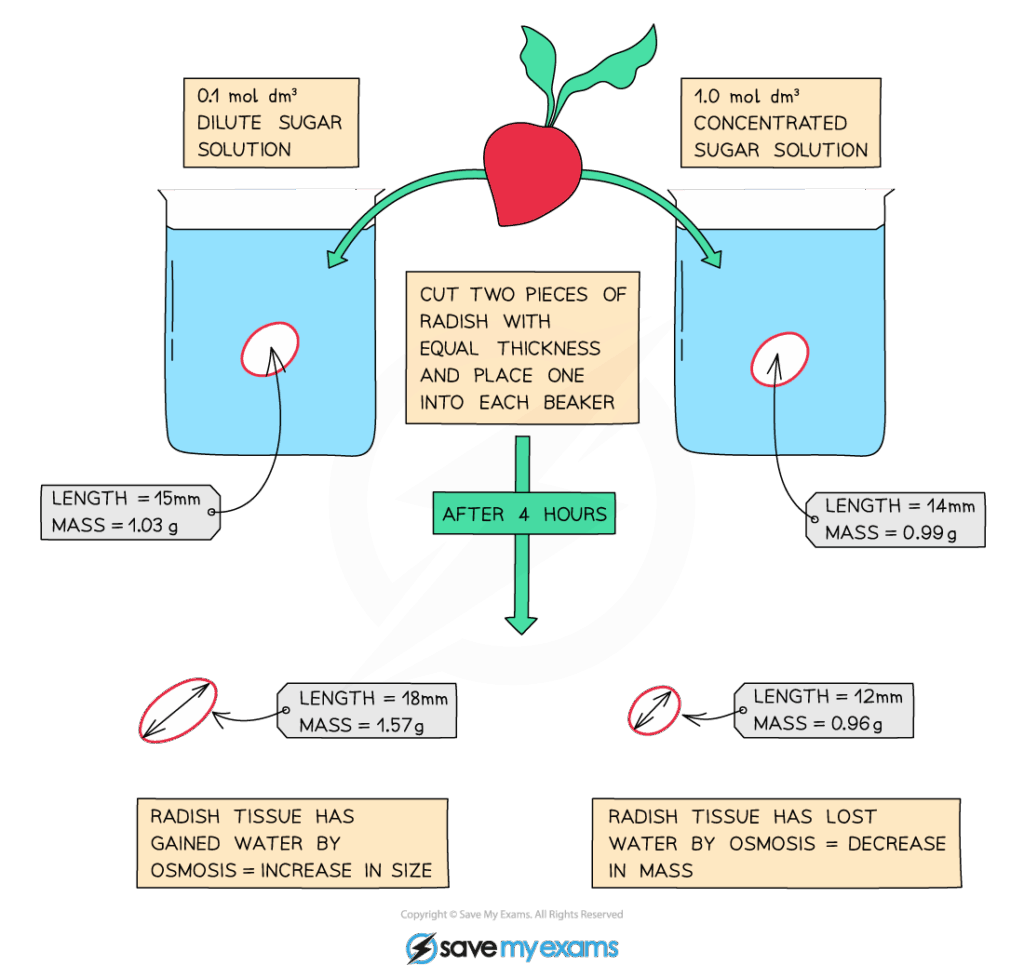
🔍 TOK Perspective: Models of osmosis simplify membranes as perfect barriers, but in reality aquaporins and active transporters complicate water movement. TOK issue: How reliable are simplified models in representing complex systems?