D1.2.3 POST-TRANSLATIONAL MODIFICATIONS
📌Definition Table
| Term | Definition |
|---|---|
| Post-translational modification (PTM) | Chemical or structural changes to a polypeptide after translation that alter its function. |
| Phosphorylation | Addition of phosphate groups to proteins, often regulating enzyme activity or signaling. |
| Glycosylation | Attachment of carbohydrate chains to proteins, important for stability and cell recognition. |
| Proteolytic cleavage | Cutting of polypeptide chains to activate or mature proteins. |
| Ubiquitination | Addition of ubiquitin tags marking proteins for degradation by proteasomes. |
| Chaperones | Proteins that assist proper folding of newly synthesized polypeptides. |
📌Introduction
Once polypeptides are synthesized by ribosomes, they are rarely functional in their raw form. Post-translational modifications (PTMs) refine protein structure, regulate activity, and target proteins to specific locations. These modifications expand the diversity of the proteome far beyond what is encoded by the genome and are central to nearly all cellular processes.
📌 Folding and Chaperones
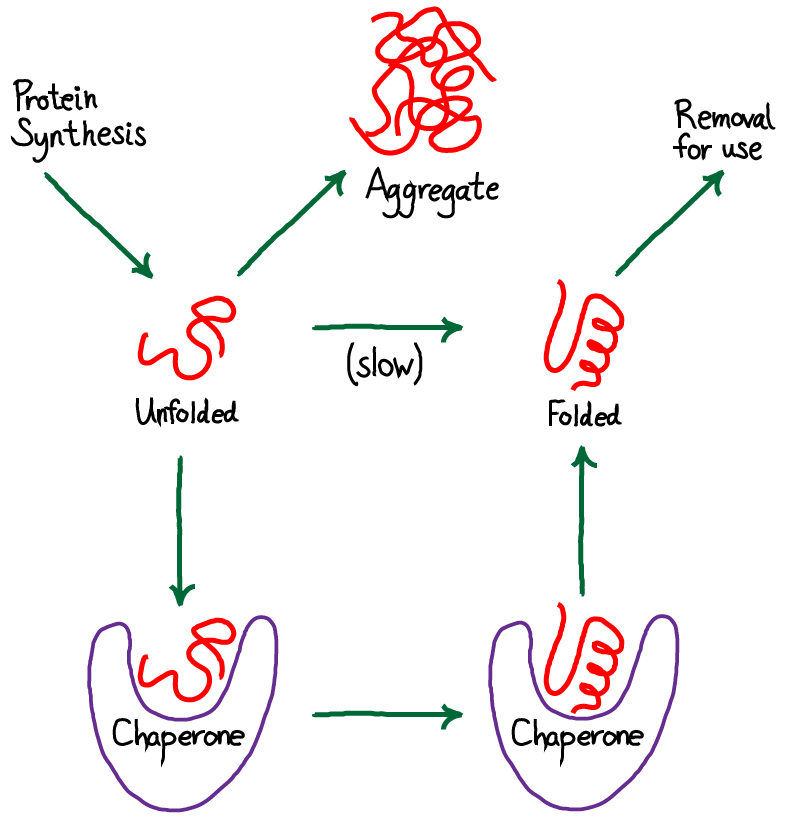
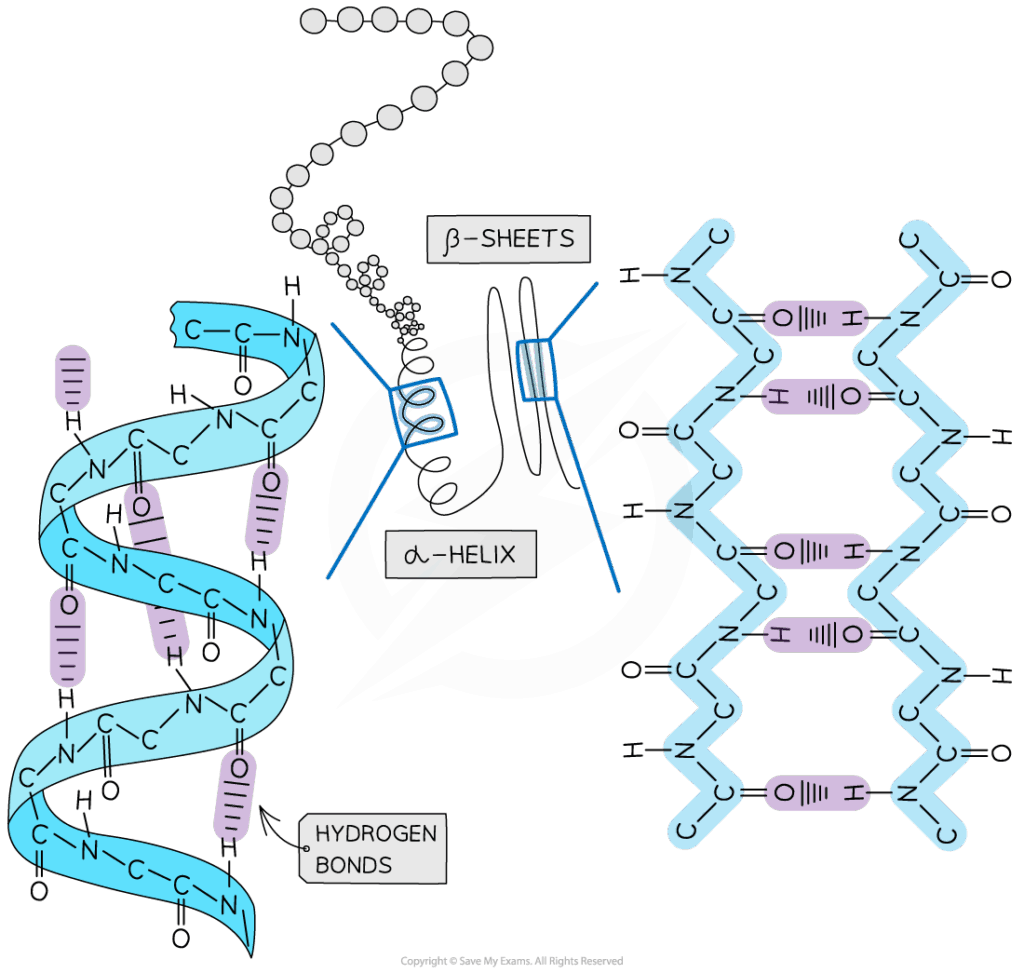
- Polypeptides must fold into precise 3D structures to function.
- Chaperone proteins prevent misfolding and aggregation during folding.
- Incorrect folding can cause diseases such as Alzheimer’s, Parkinson’s, or prion diseases.
- Protein disulfide isomerases catalyze disulfide bond formation, stabilizing structure.
- Folding often occurs in the endoplasmic reticulum (ER) for secretory proteins.
🧠 Examiner Tip: Always mention that correct folding is essential for protein function; misfolding usually results in loss of activity or toxicity.
📌 Chemical Modifications
- Phosphorylation adds phosphate groups (via kinases), switching enzymes or receptors “on” or “off.”
- Glycosylation attaches carbohydrate chains, crucial for protein stability, signaling, and immune recognition.
- Acetylation modifies histones, regulating gene expression by altering chromatin structure.
- Methylation can regulate protein interactions and epigenetic gene control.
- These modifications create dynamic regulation of protein activity.
🧬 IA Tips & Guidance: A possible IA could examine enzyme activity under conditions that mimic phosphorylation (using activators/inhibitors), linking PTMs to enzyme regulation.
📌 Proteolytic Processing
- Some proteins are produced as inactive precursors (zymogens or pro-proteins).
- Proteolytic cleavage activates them, e.g., pepsinogen → pepsin in the stomach.
- Hormones like insulin are produced as prohormones, requiring cleavage for activation.
- Viral proteins often rely on host proteases for activation, a target for antiviral drugs.
- Cleavage ensures proteins are only active at the right time and place.
🌐 EE Focus: An EE could investigate how glycosylation affects protein stability in biopharmaceuticals, or how phosphorylation regulates cell signaling in cancer biology.
📌 Protein Targeting and Degradation
- Signal peptides direct proteins to their proper cellular destinations (ER, mitochondria, nucleus).
- Ubiquitination tags damaged or unneeded proteins for degradation in proteasomes.
- Proteasome-mediated degradation maintains protein quality control.
- Autophagy recycles entire organelles and protein aggregates when necessary.
- Balance between synthesis and degradation maintains proteostasis.
❤️ CAS Link: Students could develop awareness projects showing how lifestyle choices (diet, toxins, stress) impact protein health and folding, linking cell biology to personal well-being.
🌍 Real-World Connection: Post-translational modifications are central to medicine and biotechnology. Cancer therapies target kinases involved in phosphorylation pathways. Glycosylation patterns are critical in developing monoclonal antibodies and vaccines. Misfolded proteins underlie neurodegenerative diseases such as Alzheimer’s and prion disorders, while proteasome inhibitors are used in cancer treatment. Biotechnology exploits PTMs to engineer stable therapeutic proteins with extended half-lives.
📌 Integration with Protein Function
- PTMs expand protein diversity beyond genetic coding.
- Dynamic modifications allow rapid adaptation to cellular conditions.
- Misregulation of PTMs is a hallmark of many diseases, especially cancer and neurodegeneration.
- PTMs connect directly to signaling, metabolism, gene regulation, and immunity.
- Studying PTMs provides insight into systems biology and proteome complexity.
🔍 TOK Perspective: PTMs are invisible molecular events studied using indirect methods like mass spectrometry. TOK reflection: How do we decide when indirect evidence is strong enough to establish knowledge, and does the complexity of PTMs challenge the idea of a simple central dogma?