B1.2.1 – FORMATION AND VARIETY OF PROTEINS
📌Definition Table
| Term | Definition |
|---|---|
| Amino acid | Monomer of proteins; contains an amine group (–NH₂), a carboxyl group (–COOH), a hydrogen, and an R group around a central carbon. |
| Peptide bond | Covalent bond between amino acids formed by condensation (between –COOH and –NH₂ groups). |
| Dipeptide | Molecule formed when two amino acids join via a peptide bond. |
| Polypeptide | Chain of three or more amino acids joined by peptide bonds. |
| Essential amino acids | Amino acids that must be obtained from diet because humans cannot synthesize them. |
| Non-essential amino acids | Amino acids that can be synthesized by the body. |
📌Introduction
Proteins are macromolecules essential for nearly every cellular process, from catalyzing reactions to providing structural support. They are polymers of amino acids, assembled by ribosomes under the genetic instructions encoded in DNA. The immense variety of proteins arises from the 20 amino acids that can be combined in almost infinite sequences, leading to structural and functional diversity.
📌 Formation of Polypeptides
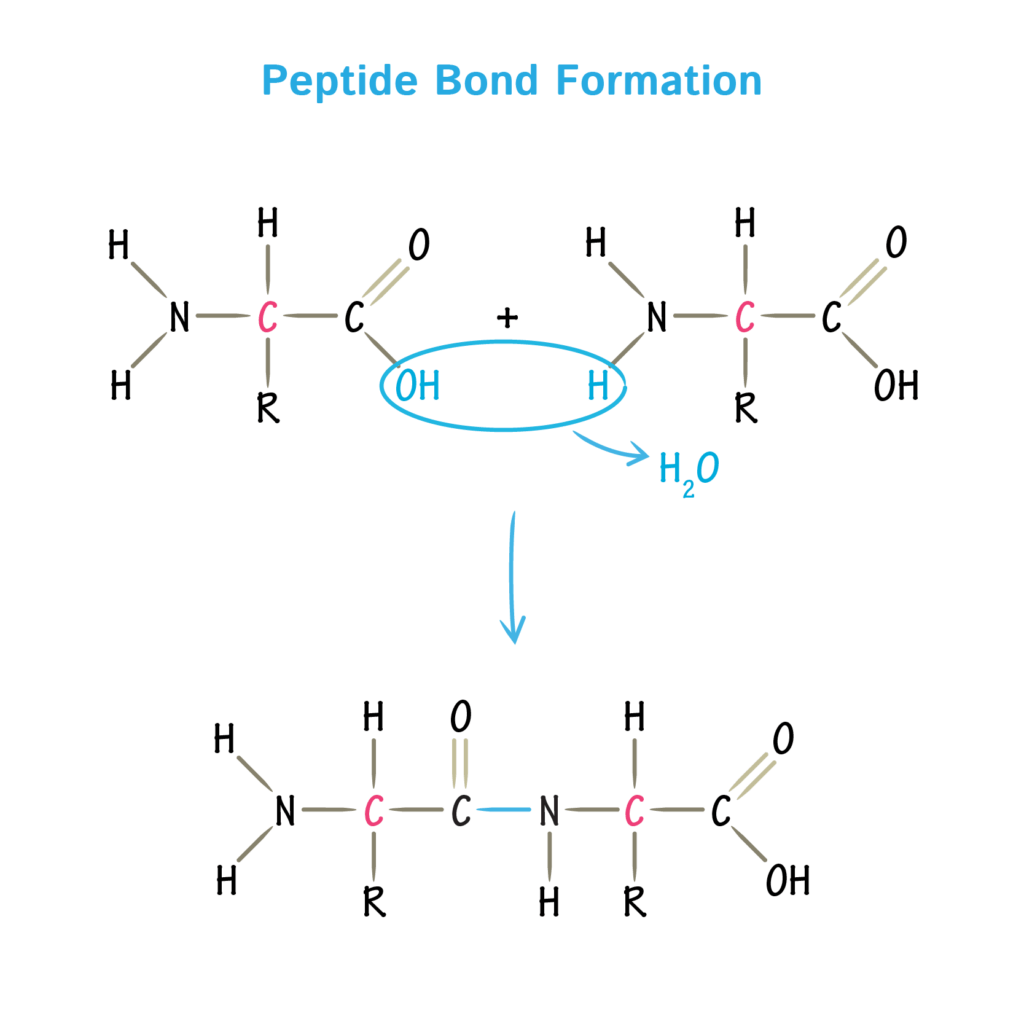
- Amino acids link together through condensation reactions, where the carboxyl group (–COOH) of one amino acid reacts with the amine group (–NH₂) of another, releasing a molecule of water and forming a peptide bond.
- The resulting bond is strong and stable, allowing long chains of amino acids (polypeptides) to form. A short chain of two amino acids is a dipeptide, while chains of many amino acids make up polypeptides, which fold into proteins.
- Proteins are synthesized by ribosomes during translation, where mRNA provides the instructions and tRNA delivers the appropriate amino acids.
- The vast number of possible sequences — even a short chain of 50 amino acids allows trillions of combinations — explains the extraordinary diversity of protein structures and functions.
- Hydrolysis reactions, catalyzed by protease enzymes, can break peptide bonds by adding water, returning proteins to their amino acid monomers.
🧠 Examiner Tip: When drawing peptide bond formation, always show the loss of water (–OH from carboxyl, –H from amine) and highlight the C–N bond that results. Make sure to note that the R group is not directly involved in bond formation but is responsible for diversity.
📌 Essential and Non-Essential Amino Acids
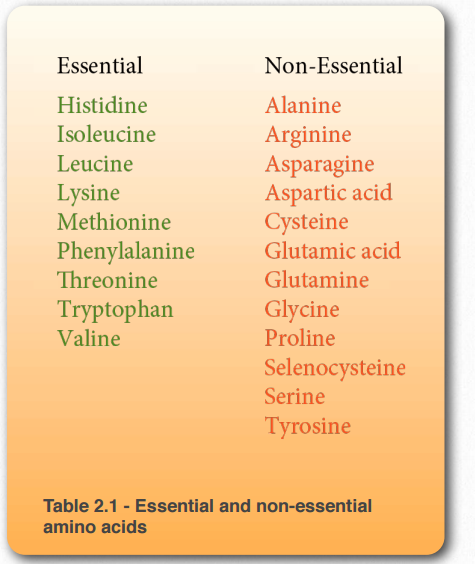
- Of the 20 amino acids used in protein synthesis, humans can synthesize 11, known as non-essential amino acids, because they can be made from other compounds within the body.
- The other 9 are essential amino acids, which must be obtained from the diet because humans lack the metabolic pathways to produce them.
- Animal-derived foods (such as meat, eggs, and dairy) are considered “complete proteins” because they contain all essential amino acids in sufficient quantities.
- Plant-based foods often lack one or more essential amino acids, but by combining different plant sources (e.g., legumes with grains), vegetarians and vegans can obtain all essentials.
- Protein deficiency, particularly a lack of essential amino acids, can lead to health conditions such as kwashiorkor, highlighting the importance of dietary variety.
🧬 IA Tips & Guidance: A simple lab experiment could involve the Biuret test, which detects peptide bonds by producing a violet color. More advanced IAs could test the action of proteases on protein-rich foods (e.g., egg white hydrolyzed into amino acids). Linking visible experimental outcomes back to molecular processes of peptide bond hydrolysis strengthens analysis.
📌 Variety of Proteins
- Protein variety is determined by the sequence, number, and type of amino acids that form each polypeptide. Even small changes in sequence can drastically alter a protein’s structure and function.
- The genetic code in DNA ultimately determines this sequence, with codons specifying which amino acids are assembled during protein synthesis.
- Proteins can be short peptides with fewer than 100 amino acids, or enormous complexes with thousands of subunits, reflecting their versatility.
- The 20 amino acids differ by their R groups, which may be hydrophobic, hydrophilic, acidic, or basic. This chemical diversity influences folding, interactions, and ultimately protein function.
- As a result, proteins can act as enzymes, transporters, receptors, structural fibers, storage molecules, hormones, or antibodies, making them indispensable to life.
🌐 EE Focus: An EE could explore how mutations in DNA alter amino acid sequence and protein function, for example in diseases like sickle-cell anemia. Another avenue would be investigating how synthetic biology manipulates amino acid sequences to create novel proteins for biotechnology.
📌 Examples of Protein Diversity
- Rubisco: The enzyme central to carbon fixation in photosynthesis; considered one of the most abundant proteins on Earth.
- Insulin: A small protein hormone secreted by the pancreas that regulates blood glucose by promoting uptake into cells.
- Immunoglobulins: Antibodies that recognize and bind to a vast range of antigens due to highly variable regions in their amino acid sequences.
- Rhodopsin: A light-sensitive receptor protein in the retina that allows vision in dim light.
- Collagen: A fibrous protein with a triple-helix structure that provides tensile strength in connective tissues, ligaments, and skin.
- Spider silk: A protein fiber produced by spiders that is both lightweight and stronger than steel, with potential for industrial and biomedical applications.
❤️ CAS Link: Students could design a nutrition awareness project comparing plant-based and animal-based diets, explaining how different protein sources provide essential amino acids and how combinations of foods can ensure balanced intake.
🌍 Real-World Connection: Proteins underpin advances in medicine, agriculture, and industry. Insulin, once harvested from animals, is now mass-produced using genetically engineered bacteria. Antibodies form the basis of diagnostic tests and treatments for infections. Industrial enzymes (e.g., proteases in detergents) improve cleaning efficiency, while collagen and keratin are used in cosmetics and tissue repair. Understanding protein diversity allows for innovation in biotechnology and healthcare.
📌 Applications in Biology
- Proteins function as enzymes, speeding up biochemical reactions that sustain life.
- As hormones, proteins such as insulin and glucagon regulate physiological processes.
- Antibodies defend organisms against pathogens by specifically binding to foreign molecules.
- Structural proteins like keratin and collagen provide mechanical support in tissues and organs.
- Transport proteins such as hemoglobin carry oxygen in the blood, while membrane channels regulate ion and nutrient movement.
🔍 TOK Perspective: The sheer diversity of proteins raises the question of reductionism in science. Although proteins are built from just 20 amino acids, predicting their three-dimensional folding and function remains one of biology’s great challenges. TOK reflection: To what extent can complex biological phenomena be fully explained by the sum of their chemical parts?