A1.1.2 – PHYSICAL AND CHEMICAL PROPERTIES OF WATER
📌Definition Table
| Term | Definition |
|---|---|
| Specific Heat Capacity | The amount of energy required to raise the temperature of 1 kg of a substance by 1°C. |
| Latent Heat of Vaporization | Energy required to convert 1 g of liquid into vapor without a temperature change. |
| Cohesion | Hydrogen bonding between water molecules, allowing them to stick together. |
| Adhesion | Attraction between water and other polar molecules or surfaces. |
| Density Anomaly | Ice is less dense than liquid water due to hydrogen bonding, so it floats. |
| Viscosity | A fluid’s resistance to flow; water has low viscosity compared to other liquids. |
📌Introduction
Water’s physical and chemical properties are unique compared to other molecules of similar size, and these are crucial for sustaining life on Earth. These properties are direct consequences of hydrogen bonding and polarity, which give water an unusually high boiling point, high specific heat capacity, high latent heat of vaporization, and the ability of ice to float. In biology, these features stabilize aquatic ecosystems, regulate body temperatures, enable plant transport, and support global climate balance.
📌 Cohesion and Adhesion
- Cohesion occurs when water molecules hydrogen bond with each other, creating a strong internal attraction.
- This allows the formation of continuous water columns in xylem, essential for transpiration pull.
- Adhesion occurs when water bonds to other polar molecules, such as cellulose in plant walls.
- Adhesion supports capillary action, enabling water to climb against gravity in narrow spaces.
- Cohesion produces surface tension, which allows organisms like pond skaters to move across water surfaces.
- Together, cohesion and adhesion maintain water flow in plants and soils, crucial for terrestrial ecosystems.
🧠 Examiner Tip: Clearly distinguish cohesion (water–water) and adhesion (water–other). This difference is often tested directly in Paper 2.
📌 Solvent Properties
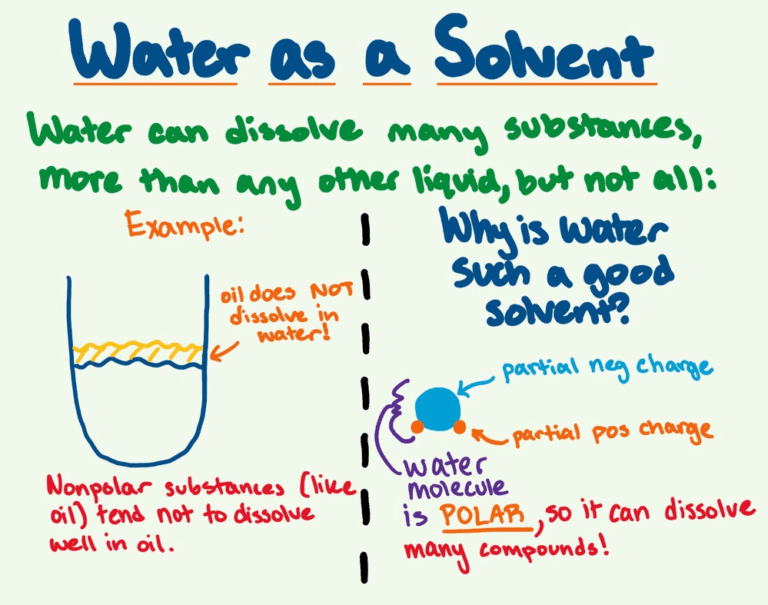
- Water’s polarity allows it to dissolve ionic compounds (NaCl → Na⁺ and Cl⁻) and polar molecules like glucose.
- This ability underpins transport of nutrients and wastes in cytoplasm, blood plasma, and plant sap.
- Hydrophobic molecules like lipids do not dissolve; instead, they cluster together (hydrophobic effect).
- The hydrophobic effect drives membrane formation and protein folding.
- Amphipathic molecules (e.g., phospholipids) arrange into bilayers spontaneously, forming the basis of cell membranes.
- Oxygen has low solubility in water, particularly at body temperature; hemoglobin overcomes this limitation in animals.
- Without solvent properties, water could not function as the medium of metabolism or transport.
🧬 IA Tips & Guidance: In osmosis, transpiration, or enzyme activity experiments, highlight how water’s solvent capacity and polarity explain the observed results. Always link back to hydrogen bonding as the cause of solubility differences.
📌 Thermal Properties (Specific Heat & Latent Heat)
- Water has a high specific heat capacity (4.2 J/g°C) due to the energy required to break hydrogen bonds.
- This buffers organisms against rapid temperature fluctuations.
- Aquatic ecosystems remain stable despite external temperature changes.
- Water also has a high latent heat of vaporization, making evaporation a powerful cooling mechanism.
- Sweating in humans and transpiration in plants remove large amounts of heat.
- Thermal stability in organisms is vital for maintaining enzyme activity at optimal temperatures.
- Oceans and lakes act as global heat sinks, moderating climate by absorbing excess energy.
🌐 EE Focus: Extended essays could investigate how heat capacity stabilizes aquatic ecosystems or how solubility and thermal properties of water affect enzyme activity.
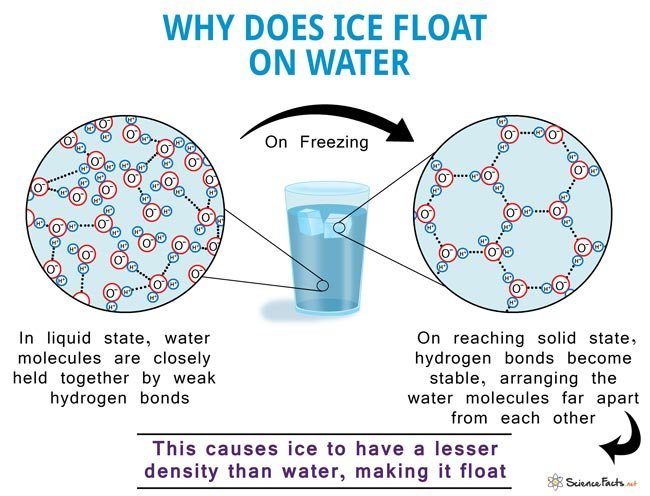
📌 Density and Ice Formation
- In liquid water, hydrogen bonds constantly break and reform, keeping molecules close together.
- As water freezes, molecules form a rigid hydrogen-bonded lattice, increasing the spacing between them.
- Ice becomes less dense than liquid water, causing it to float.
- Floating ice insulates underlying water, protecting aquatic organisms during winter.
- Ice provides seasonal habitats for organisms like seals and polar bears.
- Without this property, lakes and oceans could freeze solid, destroying ecosystems.
❤️ CAS Link: A CAS project could involve educating communities about water conservation or climate action, demonstrating how ice floating and heat buffering are critical for ecosystems and biodiversity.
📌 Other Physical Properties (Thermal Conductivity, Buoyancy, Viscosity)
- Water has higher thermal conductivity than air, helping organisms dissipate or retain heat.
- Aquatic animals use water’s buoyancy to grow larger without skeletal collapse.
- Blubber in seals and insulating feathers in diving birds adapt to water’s buoyant and thermal properties.
- Low viscosity allows efficient movement for aquatic animals like fish and diving birds.
- These physical traits explain the success of life in aquatic environments.
🌍 Real-World Connection: Water’s unique properties regulate climate, sustain ecosystems under ice, and enable agriculture through transpiration. Human survival, biodiversity, and weather patterns all rely on them.
🔍 TOK Perspective: The study of water’s properties demonstrates how unseen molecular interactions (hydrogen bonding) explain large-scale phenomena like climate regulation and plant transport. A TOK reflection could ask: How far can models of invisible forces, such as hydrogen bonding, be trusted to explain observable biological processes?