A1.1.1 – HYDROGEN BONDING AND THE BIOLOGICAL IMPORTANCE OF WATER
📌Definition Table
| Term | Definition |
|---|---|
| Hydrogen Bond | Weak electrostatic attraction between a δ⁺ hydrogen atom (bonded to an electronegative atom) and a δ⁻ atom (commonly oxygen or nitrogen) in another molecule. |
| Polarity | Unequal sharing of electrons in a covalent bond leading to regions of partial charge. |
| Dipole | A molecule with distinct regions of δ⁺ and δ⁻ charges due to polarity. |
| Cohesion | Attraction between molecules of the same type (e.g., water–water via hydrogen bonds). |
| Adhesion | Attraction between water molecules and other polar/charged substances. |
| Solvent | A liquid that dissolves solutes to form a solution; water is considered the universal solvent. |
📌Introduction
Water is the most fundamental biological molecule and is central to life processes. It constitutes 70–95% of the mass of living cells and serves as the medium for biochemical reactions, a solvent for metabolites, and a regulator of temperature. Water’s biological importance stems directly from its polarity and its capacity to form hydrogen bonds. These hydrogen bonds are individually weak but collectively powerful, giving water unique physical and chemical properties that underpin processes such as nutrient transport, enzyme catalysis, temperature stability, and macromolecular stability. Life on Earth is so dependent on water that the search for life on other planets begins with looking for its presence.
📌 Structure and Polarity of Water
- A water molecule consists of one oxygen atom covalently bonded to two hydrogen atoms in a bent shape (~104.5° bond angle).
- Oxygen’s higher electronegativity causes electrons to be shared unequally, creating δ⁻ charge on oxygen and δ⁺ charges on hydrogens.
- This results in a polar molecule with a permanent dipole moment.
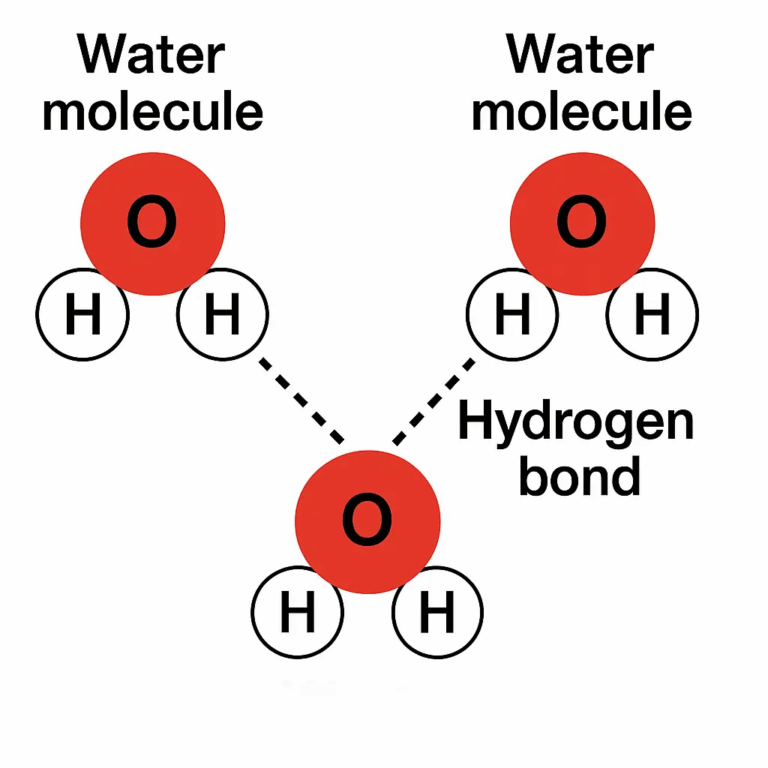
- Hydrogen bonds form between δ⁺ hydrogen of one water molecule and δ⁻ oxygen of another.
- Although weak individually (~5–30 kJ/mol), in aggregate they form a strong network stabilizing water’s structure.
- The polar nature explains water’s solvent capacity, high surface tension, and higher boiling/melting points compared to similar-sized molecules.
- The constant breaking and reforming of hydrogen bonds gives water fluidity while maintaining cohesion.
- Without polarity, water would behave more like hydrogen sulfide (H₂S), a gas at room temperature — unsuitable for life.
🧠 Examiner Tip: Always show δ⁺/δ⁻ in diagrams, use dotted lines for hydrogen bonds, and explicitly state that hydrogen bonds are intermolecular forces, not covalent bonds.
📌 Hydrogen Bonds in Biological Molecules
- DNA: Hydrogen bonds between bases stabilize the double helix; 2 bonds between A–T and 3 between G–C make DNA both stable and flexible enough for replication.
- Proteins: Secondary structures (α-helices, β-sheets) rely on intramolecular hydrogen bonds; tertiary structure is stabilized by hydrogen bonding between R-groups.
- Carbohydrates: Cellulose fibers gain tensile strength from hydrogen bonds between chains, allowing plants to stand upright.
- Structural proteins: Collagen’s triple helix is stabilized by hydrogen bonds, giving connective tissue tensile strength.
- Enzymes: Substrate binding and enzyme–substrate complexes are stabilized by hydrogen bonds, essential for catalysis.
- RNA: Hydrogen bonds mediate codon–anticodon pairing between mRNA and tRNA during translation, ensuring accurate protein synthesis.
- Membranes: Polar phosphate heads of phospholipids form hydrogen bonds with water, stabilizing bilayers and maintaining cell integrity.
- Polysaccharides and glycoproteins: Hydrogen bonding ensures solubility and recognition in cell–cell communication.
🧬 IA Tips & Guidance: Experiments on enzyme activity, DNA denaturation, or protein folding can highlight hydrogen bonding. For example, varying pH or temperature shows how disruption of hydrogen bonds alters structure and function. Always link results back to the molecular basis: breaking hydrogen bonds destabilizes biological structures.
📌 Water as a Solvent and Medium for Life
- Water’s polarity allows it to dissolve ionic compounds (NaCl dissociates into Na⁺ and Cl⁻) and polar molecules like glucose.
- This property underpins the transport of nutrients and wastes in blood plasma, plant sap, and cytoplasm.
- Nonpolar molecules (lipids, fats) do not dissolve; instead, hydrophobic clustering occurs, driving membrane and protein folding.
- Amphipathic molecules like phospholipids self-assemble into bilayers due to hydrophobic interactions, essential for membranes.
- Enzymatic reactions occur in aqueous environments where substrates and enzymes can collide freely.
- Water stabilizes enzyme conformation by forming hydration shells around charged residues.
- Oxygen has low solubility in water, especially at 37°C; hemoglobin overcomes this limitation in blood.
- Without water’s solvent properties, metabolism would not occur, and life could not persist.
🌐 EE Focus: Extended essays could explore solubility of different biomolecules, enzyme kinetics in aqueous vs non-aqueous solutions, or hydrogen bonding in protein folding. Strong research connects molecular polarity to biological function.
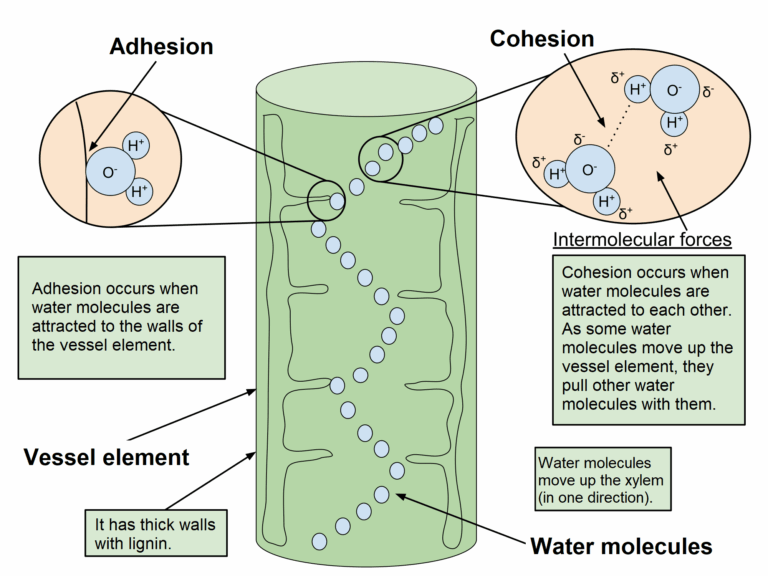
📌 Cohesion and Adhesion in Transport
- Cohesion: Hydrogen bonds cause water molecules to stick together, enabling continuous water columns in xylem.
- Cohesion underlies the cohesion–tension mechanism, explaining how water moves against gravity during transpiration.
- Adhesion: Water molecules form hydrogen bonds with cellulose in xylem walls, supporting capillary action.
- This adhesion prevents water columns from collapsing under tension.
- The combined effect of cohesion and adhesion maintains transpiration pull, vital for water and mineral transport in tall plants.
- Surface tension, also a product of cohesion, allows small organisms like pond skaters to move across water surfaces.
- Capillary action in soils also depends on adhesion, enabling plants to draw water from small pores.
- These forces are essential for terrestrial plant survival and global water cycling.
❤️ CAS Link: Students could build simple capillary tube models or hydroponic systems to demonstrate cohesion and adhesion, linking IB biology to sustainable farming or community awareness projects.
📌 Hydrogen Bonding and Thermal Stability
- Water has an unusually high specific heat capacity (4.2 J/g°C), buffering organisms against rapid temperature change.
- Aquatic habitats remain thermally stable, supporting biodiversity.
- High latent heat of vaporization makes evaporation an efficient cooling mechanism (sweating, transpiration).
- Ice is less dense than liquid water due to hydrogen bond lattice → floats and insulates aquatic life below.
- Hydrogen bonds require significant energy input to break, so water remains liquid across a wide temperature range.
- Oceans act as heat sinks, moderating global climate by absorbing and redistributing heat.
- Within organisms, thermal buffering maintains enzyme activity at near-optimal conditions.
- Hydrogen bonding makes water a uniquely stable medium compared to other solvents.
🌍 Real-World Connection: Global climate stability, human thermoregulation, polar ecosystems, and even weather systems all depend on hydrogen bonding in water.
📌 Water’s Role in the Origin and Continuation of Life
- Early Earth’s oceans, possibly near hydrothermal vents, provided an aqueous medium for prebiotic chemistry.
- Water dissolved molecules and allowed collisions → promoted synthesis of organic compounds.
- Encapsulation of solutes in primitive membranes within water led to protocells.
- Hydrogen bonding stabilized macromolecules like RNA and proteins, enabling catalysis.
- Water continues to support life as the solvent of metabolism, a participant in hydrolysis and condensation, and the medium of transport.
- Processes like respiration, photosynthesis, and digestion all require water.
- Search for extraterrestrial life focuses on finding liquid water, as it remains the best indicator of habitability.
- Without hydrogen bonding, water would lack the properties that made life’s origin possible.
🔍 TOK Perspective: Hydrogen bonding cannot be directly observed but is inferred from indirect evidence (boiling point anomalies, solubility, structural stability). This raises questions about how reliable indirect evidence is in building scientific knowledge. TOK reflection could explore: Is the presence of water sufficient evidence for life, or must we distinguish between necessary and sufficient conditions for life’s existence?