A2.3.2 – VIRUS REPLICATION
📌Definition Table
| Term | Definition |
|---|---|
| Lytic Cycle | Viral replication process in which the host cell is lysed to release new virus particles. |
| Lysogenic Cycle | Viral replication strategy in which the viral genome integrates into the host genome and remains dormant before activation. |
| Reverse Transcriptase | Enzyme used by retroviruses to convert RNA into DNA inside the host cell. |
| Provirus | Viral DNA integrated into the host’s genome during lysogeny. |
| Host Range | The variety of organisms or cell types a virus can infect, determined by receptor compatibility. |
📌Introduction
Viruses cannot reproduce independently and must use the machinery of a host cell to replicate. The replication process varies depending on the virus type, genome composition, and host. Two main replication pathways are observed in bacteriophages: the lytic cycle, which rapidly destroys the host cell, and the lysogenic cycle, which allows the virus to persist in a dormant state before activation. Animal viruses may enter via membrane fusion or endocytosis, while bacteriophages inject their DNA directly. Retroviruses like HIV have unique replication mechanisms involving reverse transcription.
📌 The Lytic Cycle
- Virus attaches to host cell via specific receptor proteins.
- Viral genome enters the cell (injection for bacteriophages, fusion/endocytosis for animal viruses).
- Host cell machinery is hijacked to replicate viral nucleic acids and synthesise viral proteins.
- New viral particles are assembled in the cytoplasm.
- Host cell bursts (lysis), releasing many new viruses to infect other cells.
- This cycle results in rapid spread but also rapid destruction of host cells.
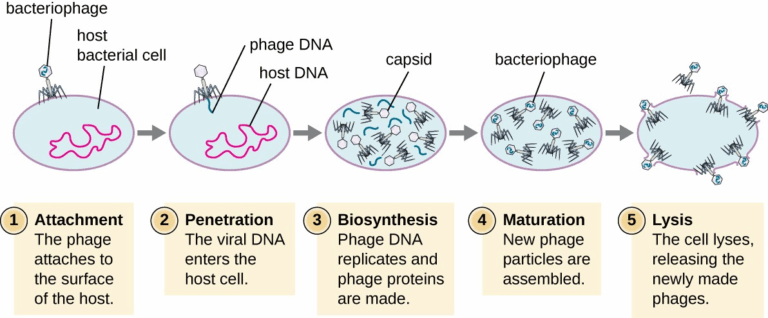
🧠 Examiner Tip: For IB diagrams, ensure the lytic cycle steps are in the correct order and labelled clearly with terms like “assembly” and “lysis”.
📌 The Lysogenic Cycle
- Viral genome integrates into host DNA, forming a provirus (or prophage in bacteria).
- The provirus is replicated along with the host’s DNA during cell division.
- No immediate damage occurs to the host cell.
- Environmental triggers (e.g., UV light, stress) can activate the virus.
- The provirus then enters the lytic cycle, producing new viruses.
- Lysogeny allows long-term persistence within the host population.
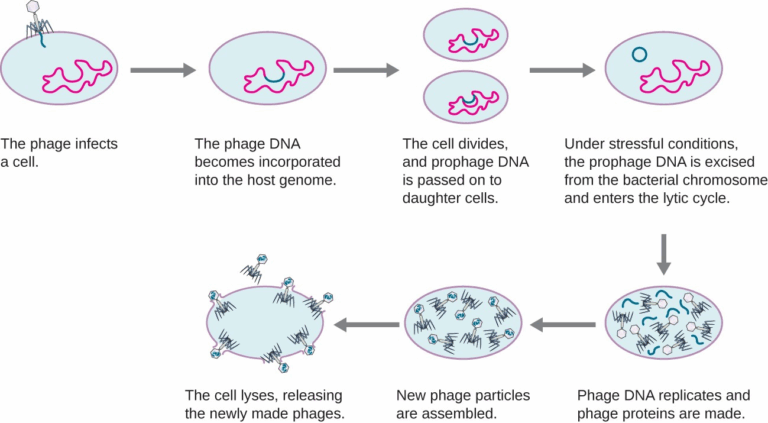
🧬 IA Tips & Guidance: A safe simulation IA could model virus spread using computer software, comparing lytic and lysogenic replication rates.
📌 Retrovirus Replication (HIV Example)
- Virus binds to specific receptors (e.g., CD4 and CCR5 on helper T cells).
- Viral RNA and enzymes enter the host cell.
- Reverse transcriptase converts viral RNA into DNA.
- Viral DNA integrates into the host genome as a provirus.
- Host cell transcribes and translates viral genes to make new viral proteins.
- New virions assemble and bud from the host cell, often without immediate lysis.
- Antiretroviral drugs target reverse transcriptase or other replication steps.
🌐 EE Focus: An EE could investigate the effectiveness of different antiviral drugs in inhibiting specific stages of viral replication.
📌 Factors Affecting Replication Speed
- Type of genome: RNA viruses generally replicate faster than DNA viruses.
- Host cell type: actively dividing cells often support faster replication.
- Immune system status: weakened immunity can allow faster viral spread.
- Presence of antiviral drugs: can slow or block replication steps.
- Environmental triggers: can activate dormant lysogenic viruses.
- Viral load: higher initial dose can lead to faster onset of symptoms.
❤️ CAS Link: A CAS project could involve designing an educational game that simulates the spread of lytic vs lysogenic viruses.
📌 Microscopy and Molecular Tools in Studying Replication
- TEM visualises viral assembly stages inside host cells.
- Fluorescent tagging tracks viral protein movement in real time.
- PCR detects and quantifies viral genomes in infected cells.
- Western blotting identifies viral proteins during replication.
- Cell culture allows controlled study of infection cycles.
- Data from these methods support antiviral drug development.
🔍 TOK Perspective: The study of viral replication shows how models and simulations help scientists understand processes too small or rapid to observe directly.
🌍 Real-World Connection:
Understanding viral replication cycles informs vaccine schedules, antiviral treatments, and strategies to control outbreaks, such as timing antiviral administration to block early replication.