A1.1.2 – PHYSICAL AND CHEMICAL PROPERTIES OF WATER
📌 Definition Table
| Term | Definition |
|---|---|
| Cohesion | The attraction between water molecules due to hydrogen bonding. |
| Adhesion | The attraction between water molecules and other polar or charged surfaces. |
| Specific Heat Capacity | The amount of energy required to raise the temperature of 1 kg of a substance by 1°C. |
| Latent Heat of Vaporization | The amount of energy required for a substance to change from liquid to gas. |
| Viscosity | The resistance of a fluid to flow. |
| Thermal Conductivity | The ability of a substance to conduct heat. |
📌Introduction
Water has a set of unique physical and chemical properties that make it ideal for supporting life. These include its cohesive and adhesive behaviour, thermal stability, solvent capabilities, and low density of ice. Most of these properties stem from water’s polarity and hydrogen bonding, making it a critical component in temperature regulation, transport, metabolism, and survival of organisms in various habitats.
❤️ CAS Link: Design a service project where students build simple capillary tube demonstrations or a hydroponic system. Use it to explain cohesion and adhesion while promoting sustainable agriculture and plant care awareness.
📌 Cohesion and Adhesion
- Cohesion results from hydrogen bonds between water molecules, allowing them to stick together.
- This creates surface tension, allowing some organisms (like pond skaters) to walk on water.
- Cohesion enables the formation of continuous water columns in plant xylem.
- Adhesion allows water to bind to polar surfaces like cellulose in plant cell walls.
- The combination of cohesion and adhesion drives capillary action, helping water move through narrow tubes.
- These forces are vital for water transport in plants during transpiration.
🧠 Examiner Tip: Use “hydrogen bonding” to explain both cohesion and adhesion, and relate them to plant transport or surface tension in short-answer questions.
📌 Water as a Solvent
- Water’s polarity allows it to dissolve ionic and polar compounds such as salts, sugars, and amino acids.
- Substances that dissolve in water are called hydrophilic, while non-polar substances are hydrophobic.
- Water forms hydration shells around ions, separating and stabilizing them in solution.
- It enables transport of dissolved substances in blood, lymph, and plant sap.
- Most biochemical reactions occur in aqueous solutions due to water’s solvent nature.
- Water facilitates interactions between molecules, making it a medium for metabolism.
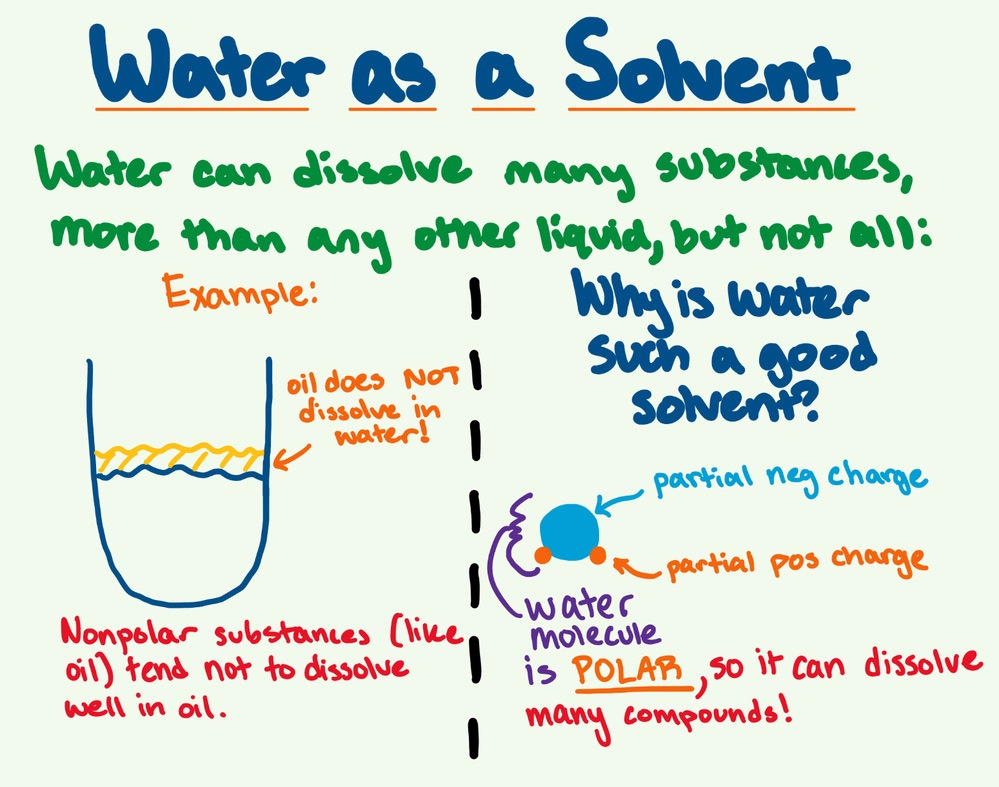
🧬 IA Tips & Guidance: When investigating solubility or reaction rates, discuss how water’s polarity and hydrogen bonding influence molecular interactions and transport mechanisms.
📌 Specific Heat Capacity
- Water has a high specific heat capacity of 4200 J/kg/°C, which means it resists rapid temperature changes.
- This stability is due to hydrogen bonds absorbing heat before increasing molecular motion.
- It helps maintain stable aquatic environments despite fluctuating air temperatures.
- Organisms can maintain homeostasis as water buffers against heat gain/loss.
- Aquatic animals benefit from minimized temperature extremes in water bodies.
- This is especially important for enzyme function, which depends on optimal temperatures.
🌐 EE Focus: An EE could investigate how water’s thermal properties influence organism survival or biochemical reaction rates in different temperature conditions.
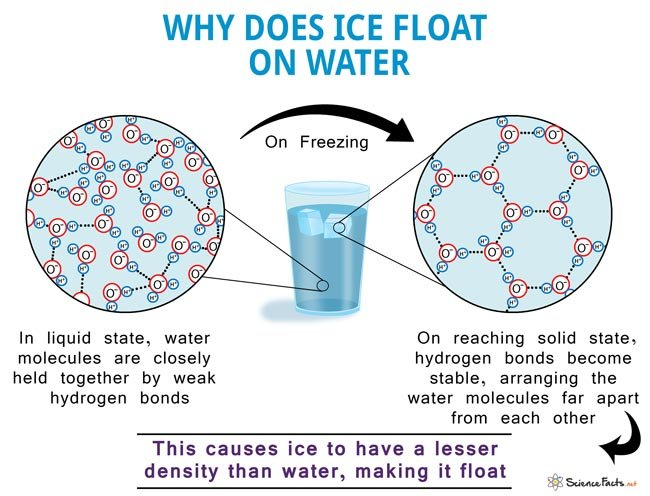
📌 Density of Ice and Buoyancy
- Ice is less dense than liquid water due to hydrogen bonding creating a structured crystal lattice.
- This makes ice float, insulating the water below and protecting aquatic life in winter.
- Floating ice forms stable platforms for animals like seals and polar bears.
- This property contributes to the thermal stability of oceans and lakes.
- Aquatic organisms survive harsh winters because water beneath the ice remains liquid.
- Buoyancy is also affected by body composition; animals like seals use blubber for buoyancy and insulation.
❤️ CAS Link: Develop a CAS project involving ecosystem protection of aquatic life or education about climate change’s impact on ice habitats.
📌 Viscosity and Movement
- Water has a low viscosity compared to other fluids, enabling efficient flow through vessels.
- This allows blood, lymph, and xylem sap to move with minimal resistance.
- Organisms like fish and aquatic birds are adapted to water’s viscosity for smooth movement.
- Water’s viscosity supports buoyant force, helping organisms float or swim.
- Streamlined body shapes evolve to reduce drag and move efficiently through water.
- Lower viscosity compared to oil or honey makes water ideal for internal circulation systems.
🌍 Real-World Connection: Water’s physical properties are crucial in engineering, medical fields (IV fluids), and climate science. Its viscosity and heat properties are considered in designing life support and artificial habitats.
📌 Thermal Conductivity and Latent Heat
- Water has relatively high thermal conductivity, helping distribute heat efficiently in organisms and environments.
- This property supports internal temperature regulation and even heat distribution in multicellular organisms.
- Water has a high latent heat of vaporization, requiring significant energy to evaporate.
- This allows for effective cooling mechanisms such as sweating or transpiration.
- Ice, due to stable hydrogen bonds, forms a lattice structure that insulates water beneath.
- These features make water excellent for thermoregulation and habitat stability.
🔍 TOK Perspective: How do we “know” water is essential for life? Are our definitions of life biased by Earth-based systems and water-dependent models?