B1.2.1 – FORMATION AND VARIETY OF PROTEINS
📌Definition Table
| Term | Definition |
|---|---|
| Amino Acid | Organic molecule containing an amino group, carboxyl group, hydrogen, and R-group. |
| Peptide Bond | Covalent bond formed between two amino acids during condensation. |
| Polypeptide | Chain of amino acids linked by peptide bonds. |
| Essential Amino Acid | Amino acid that must be obtained from the diet because the body cannot synthesise it. |
| Non-Essential Amino Acid | Amino acid that can be synthesised by the body. |
| Condensation Reaction | Chemical reaction that joins monomers, releasing water. |
📌Introduction
Proteins are polymers of amino acids, each with unique R-groups that determine the protein’s shape and function. The sequence of amino acids is coded for by mRNA, meaning protein variety is directly linked to genetic diversity. Different combinations of the 20 amino acids allow for millions of possible protein structures and functions.
❤️ CAS Link: Run a school science club workshop where students model amino acids and build polypeptides with ball-and-stick kits to demonstrate condensation reactions.
📌 Amino Acids as Protein Monomers
- Amino acids have a central carbon atom bonded to an amino group (–NH₂), carboxyl group (–COOH), hydrogen, and a variable R-group.
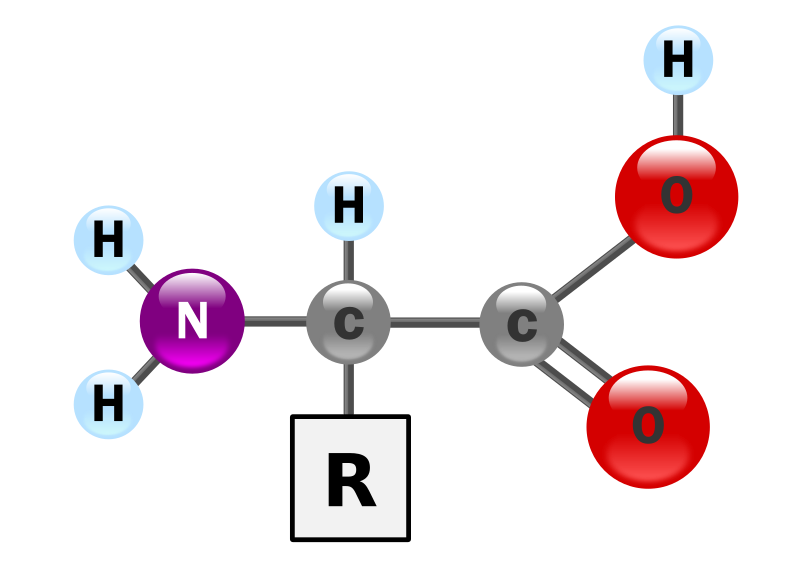
- The R-group determines chemical properties (polar, nonpolar, acidic, basic).
- There are 20 naturally occurring amino acids in the genetic code.
- Some amino acids have unique features (e.g., cysteine forms disulphide bonds).
- Classification: essential (must be obtained in diet) and non-essential (synthesised by the body).
- Amino acids are amphoteric — can act as both acids and bases.
🧠 Examiner Tip: When drawing amino acids, always show the central carbon with four bonds — missing the hydrogen or R-group loses accuracy marks.
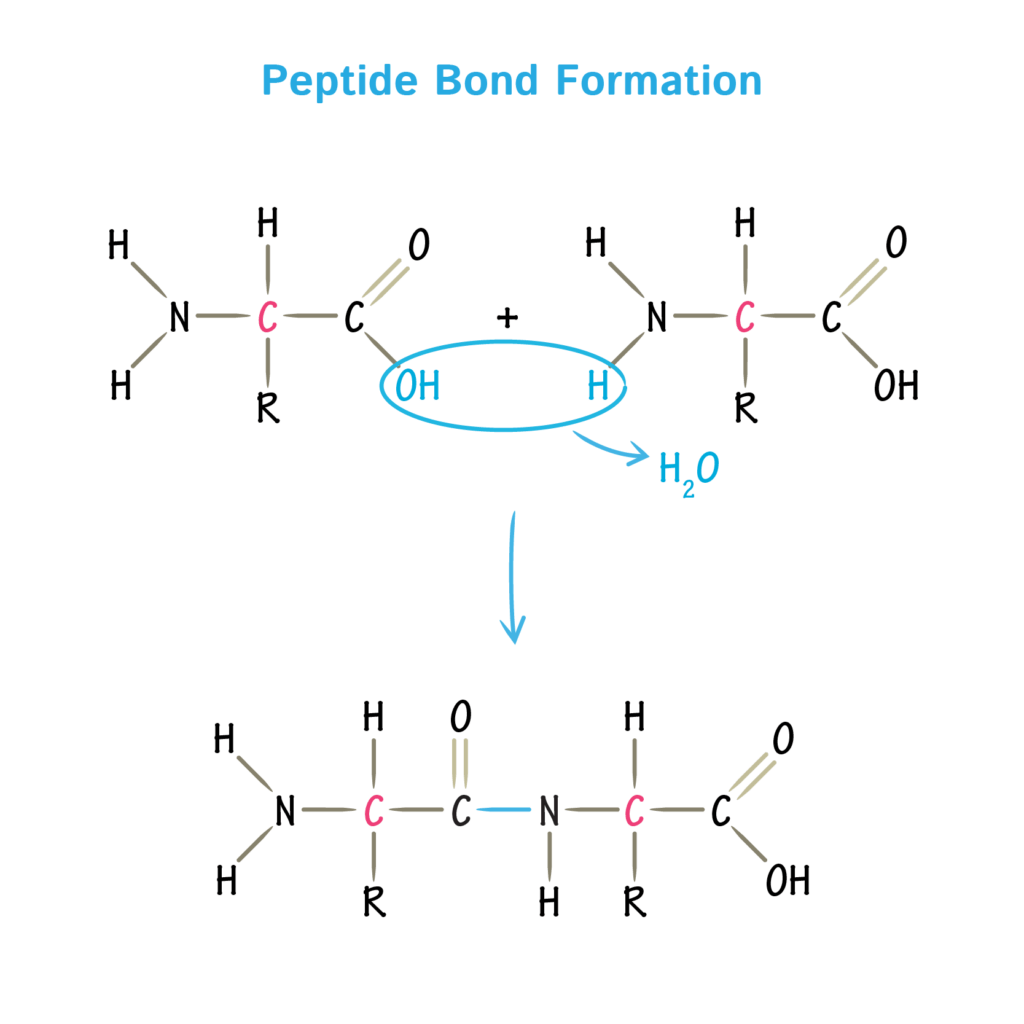
📌 Formation of Peptide Bonds
- Formed in condensation reactions between the carboxyl group of one amino acid and the amino group of another.
- Results in a covalent peptide bond and release of a water molecule.
- Reaction catalysed by ribosomes during translation.
- Hydrolysis reactions break peptide bonds with the addition of water.
- Sequence of amino acids (primary structure) is determined by the mRNA codon sequence.
- Small peptides (dipeptides, tripeptides) can have biological functions (e.g., hormones, neurotransmitters).
🌍 Real-World Connection: The artificial synthesis of human insulin involves producing the exact amino acid sequence coded by the human INS gene, then folding it to match natural insulin function.
📌 Diversity of Polypeptides
- Polypeptides can be as short as a few amino acids or over 30,000 amino acids long.
- Even small changes in sequence can cause major functional changes (e.g., sickle-cell haemoglobin mutation).
- Different combinations of amino acids give rise to proteins with unique shapes and chemical properties.
- Proteins may be made of a single polypeptide or multiple polypeptide subunits.
- Post-translational modifications (e.g., glycosylation, phosphorylation) further increase diversity.
- Structural diversity enables proteins to act in catalysis, signalling, transport, structural support, and defence.