6.4 STRATOSPHERIC OZONE
📌 Definitions Table
| Term | Definition (Exam-Ready, 2 Marks) |
|---|---|
| Soil Fumigation | The process of applying gaseous pesticides to soil to eliminate pests, pathogens, and weeds before planting. |
| Flame Retardants | Chemicals added to materials to slow the spread of fire; some types can release toxic pollutants into the atmosphere. |
| CFCs (Chlorofluorocarbons) | Synthetic compounds once used in refrigeration and aerosols that deplete the ozone layer and contribute to global warming. |
| HCFCs (Hydrochlorofluorocarbons) | Ozone-depleting substances used as transitional replacements for CFCs, with lower but still harmful ozone impact. |
- 🧠 Exam Tips:
For CFCs and HCFCs, always mention their role in ozone depletion and connection to the Montreal Protocol if asked for policy context.
For soil fumigation, link to agriculture and atmospheric pollution when relevant.
📌 UV Radiation
- The Sun emits electromagnetic radiation in a range of wavelengths, from low-frequency radio waves to high-frequency gamma radiation
- Shorter wavelengths of radiation have higher frequencies
- More energy damages living organisms
- E.g. ultraviolet (UV) radiation
Effects on human health
- Ultraviolet radiation from the Sun can have damaging effects on humanliving tissues
- When excessive UV radiation reaches the surface of the Earth, it can lead to various health issues by damaging cells and tissues
UV Radiation Effects on Humans
| Health issues caused by UV radiation | Explanation |
|---|---|
| Cataracts | Prolonged exposure to UV radiation can contribute to the development of cataractsCataracts cause clouding of the lens in the eye, leading to blurry vision and eventual vision loss if left untreated |
| UV radiation affects cells | UV radiation has the potential to induce mutations in DNA during cell divisionWhen cells are exposed to UV radiation, it can lead to genetic alterations and mutationsThis can disrupt normal cell growth and increase the risk of developing cancer |
| Skin cancer | UV radiation is a major risk factor for the development of skin cancerUV rays can damage the DNA in skin cells, leading to uncontrolled cell growth and the formation of cancerous tumoursProlonged or intense exposure to UV radiation, especially without proper protection, increases the risk of developing skin cancer |
| Sunburn | When the skin is exposed to excessive UV rays, it triggers an inflammatory response as a defence mechanismSunburned skin becomes red, painful and may blister, indicating damage to the skin cells |
| Premature skin ageing | Chronic exposure to UV radiation accelerates the ageing process of the skinIt can cause the breakdown of collagen and elastin fibres, leading to wrinkles, sagging skin and the development of age spots |
Effects on biological productivity
- Harmful UV radiation reaching the Earth’s surface affects plant growth and productivity
- Increased UV exposure can lead to:
- Reduced photosynthesis rates
- Altered plant metabolism
- Decreased crop yields
- Exposure to increased UV radiation can affect other photosynthetic organisms, such as phytoplankton
- Phytoplankton play a crucial role in aquatic food webs
- They convert sunlight, carbon dioxide and nutrients into organic matter through photosynthesis
- UV radiation damages phytoplankton by:
- Causing DNA damage
- Reducing photosynthetic activity and growth
- This leads to a decrease in primary productivity in aquatic ecosystems
- Reduced phytoplankton productivity can have cascading effects on higher trophic levels in aquatic ecosystems
- Zooplankton, which feed on phytoplankton, have less food available
- This affects their growth and reproduction
- This, in turn, can impact higher-level consumers, such as fish and marine mammals
- Organisms in these higher trophic levels rely on phytoplankton and zooplankton as food source
- This can significantly reduce the biodiversity of aquatic ecosystems
- Zooplankton, which feed on phytoplankton, have less food available
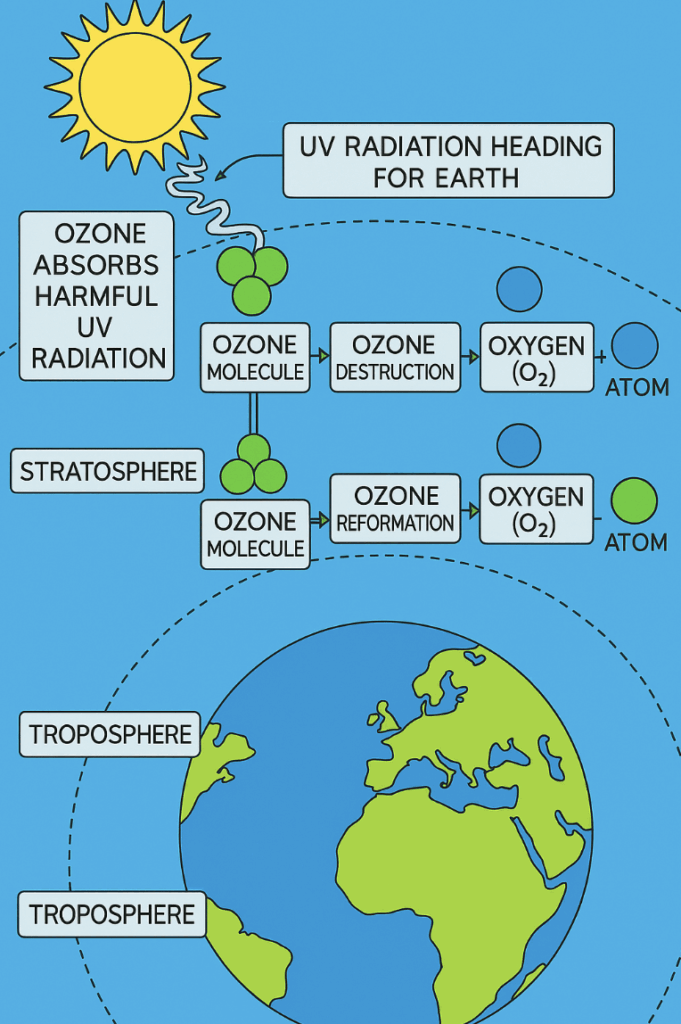
📌 The Stratospheric Ozone
- Ozone is a molecule composed of three oxygen atoms (O3)
- It is mainly found in the Earth’s stratosphere
- This is a layer of the atmosphere located approximately 10 to 50 kilometres above the Earth’s surface
- Ozone plays a very important role in protecting life on Earth
- This is because it absorbs a significant portion of the Sun’s harmful UV radiation
- This significantly reduces the amount of UV radiation that reaches the Earth’s surface
- Types of UV radiation:
- UVA:
- Longest wavelength
- Least harmful but can cause skin aging and contribute to skin cancer
- UVB:
- Medium wavelength
- Can cause skin burns and direct DNA damage
- Mostly absorbed by stratospheric ozone, but some reaches the Earth’s surface
- UVC:
- Shortest wavelength
- Most harmful
- Completely absorbed by stratospheric ozone
- UVA:
Ozone equilibrium
- The amount of ozone in the stratosphere remains relatively constant over long periods
- This is due to a steady state of equilibrium
- Equilibrium is maintained between the processes of ozone formation and destruction
- When UV radiation from the Sun interacts with ozone molecules, some of the ozone absorbs the energy and breaks apart
- This results in the formation of an oxygen molecule (O2) and a free oxygen atom (O)
- This process of ozone destruction occurs naturally in the stratosphere
- Under normal conditions, the free oxygen atom (O) can combine with another oxygen molecule (O2) to form ozone (O3) again
- This ozone destruction and reformation creates a dynamic equilibrium in the stratosphere
- There is a continuous cycle of ozone molecules being broken apart and reformed
- This dynamic equilibrium ensures that the concentration of ozone in the stratosphere remains relatively stable over time
- The rate of the forward reaction equals the rate of the backward reaction in the system, so the concentrations of the reactants and products remain relatively constant
Ozone-depleting substances
- Ozone-depleting substances (ODSs) are chemicals that cause stratospheric ozone depletion
- These substances cause the destruction of ozone molecules
- In other words, they enhance the natural ozone breakdown process (beyond natural levels)
- ODSs are commonly used in various human activities and products:
Sources of Ozone Depleting Substances
| Source | Details |
|---|---|
| Aerosols | Chlorofluorocarbons (CFCs) were previously used as propellants in aerosol products like sprays, foams, and deodorantsWhen released into the atmosphere during spraying, these substances can eventually reach the stratosphere and contribute to ozone depletion |
| Gas-blown plastics | ODSs were also used as blowing agents in the production of foamed plasticsThese agents help create air pockets within the plastic material, making it lightweightDuring manufacturing or disposal of these products, ODSs can be released into the atmosphere |
| Pesticides | Some pesticides, e.g. those containing methyl bromide, have been used in agricultural practices for soil fumigationWhen applied, these substances can vaporise and enter the atmosphere, where they can contribute to ozone depletion |
| Flame retardants | Some flame retardants contain halogen atoms and have been used in various products to reduce their flammabilityWhen these products degrade or are disposed of, the halogenated compounds can be released into the atmosphere |
| Refrigerants | ODSs were widely used as refrigerants in cooling systems, such as air conditioners and refrigeratorsThe most well-known examples are CFCsWhen these refrigerants leak or are improperly disposed of, they can reach the stratosphere and contribute to ozone depletion |
Imbalance in equilibrium
- When ozone formation and destruction rates are unequal, equilibrium is disrupted
- This leads to increased ozone depletion
- Increased UVB radiation reaches the Earth’s surface
- Affects ecosystems and human health
- Causes increased rates of skin cancer and cataracts
- Reduces terrestrial and marine productivity
Ozone holes
- Ozone depletion affects the entire Earth’s stratosphere
- However, ozone holes are most prominent at the poles
- Ozone holes are areas of low stratospheric ozone
- These holes appear every spring due to ODSs and seasonal weather patterns
❤️ CAS Tip: Create an educational video or exhibition on climate change and ozone depletion for younger students.
📌 The Montreal Protocol
The role of UNEP
- The United Nations Environment Programme (UNEP) has played a critical role in the protection of the stratospheric ozone layer
- This have been achieved through its efforts in providing information and creating international agreements:
- UNEP has been instrumental in raising awareness about:
- The fact that the ozone layer was being rapidly depleted
- The causes of this depletion
- The associated environmental and health impacts of this depletion
- Through research and sharing of information, UNEP has helped educate governments, industries and the public about the importance of ozone layer protection
- UNEP has been actively involved in the creation of international agreements aimed at reducing the use of ozone-depleting substances (ODSs)
- The Montreal Protocol on Substances that Deplete the Ozone Layer was initiated in 1987
- It was started under the guidance of UNEP
- It is a landmark international agreement that regulates the production, trade and use of chlorofluorocarbons (CFCs) and other ODSs.
- 24 countries initially signed the initial protocol, and the total now stands at 197 countries
- It has been updated and strengthened (a later amendment at a summit in Copenhagen in 1992 tightened restrictions further)
- It has resulted in emissions of ODSs falling rapidly from around 1.5 million tonnes in 1987 to around 400 000 tonnes in 2010
- UNEP hopes to end production of all HCFCs by 2040
- The illegal market for ozone-depleting substances is a significant challenge to the effectiveness of ozone protection efforts:
- UNEP recognises the need for consistent monitoring and enforcement to tackle this issue
- By collaborating with national authorities, customs agencies and other relevant stakeholders, UNEP works towards:
- Stopping the illegal trade of ozone-depleting substances
- Ensuring compliance with international regulations
- Phased reductions:
- Gradual reduction schedules for ODSs have allowed industries to adapt
- The Montreal Protocol provided time for the development and adoption of alternatives to ODSs
- National governments play an important role in implementing the agreements made by the UNEP:
- In response to the Montreal Protocol, governments have enacted national laws and regulationsto decrease the consumption and production of halogenated organic gases, such as chlorofluorocarbons (CFCs)
- These laws help enforce the reduction targets and promote the transition to ozone-friendly alternatives
- The collective efforts of UNEP, governments, industries and other stakeholders are vital in achieving goals, including:
- Ozone layer protection
- Mitigating the illegal trade of ozone-depleting substances
- Encouraging global cooperation for a more sustainable future
Planetary boundary for stratospheric ozone depletion
- Stratospheric ozone depletion is one of the nine planetary boundaries outlined by the planetary boundaries model
- Planetary boundaries are thresholds that lead to significant environmental changes if they are crossed
- The Montreal Protocol is regarded as the most successful example yet of international cooperation in management and intervention to resolve a significant environmental issue
- Actions taken in response to the Montreal Protocol have prevented the planetary boundary for stratospheric ozone depletion being crossed
- Evidence from data:
- Data shows a decrease in the size of ozone holes over time
- Continuous monitoring indicates that ozone layer recovery is underway