D2.2.2 EPIGENETIC REGULATION
📌Definition Table
| Term | Definition |
|---|---|
| Epigenetics | Heritable changes in gene expression not caused by changes in DNA sequence. |
| DNA methylation | Addition of methyl groups to cytosine bases, usually silencing genes. |
| Histone modification | Chemical changes to histone proteins that alter chromatin structure and gene expression. |
| Chromatin | Complex of DNA and proteins that packages genetic material. |
| Euchromatin | Loosely packed, transcriptionally active DNA. |
| Heterochromatin | Densely packed, transcriptionally inactive DNA. |
📌Introduction
Epigenetics provides an extra layer of gene regulation beyond DNA sequence. Chemical modifications to DNA and histones affect chromatin accessibility, determining whether genes are expressed or silenced. These changes can be inherited but are also reversible, making them critical in development, cell differentiation, and disease
📌 DNA Methylation
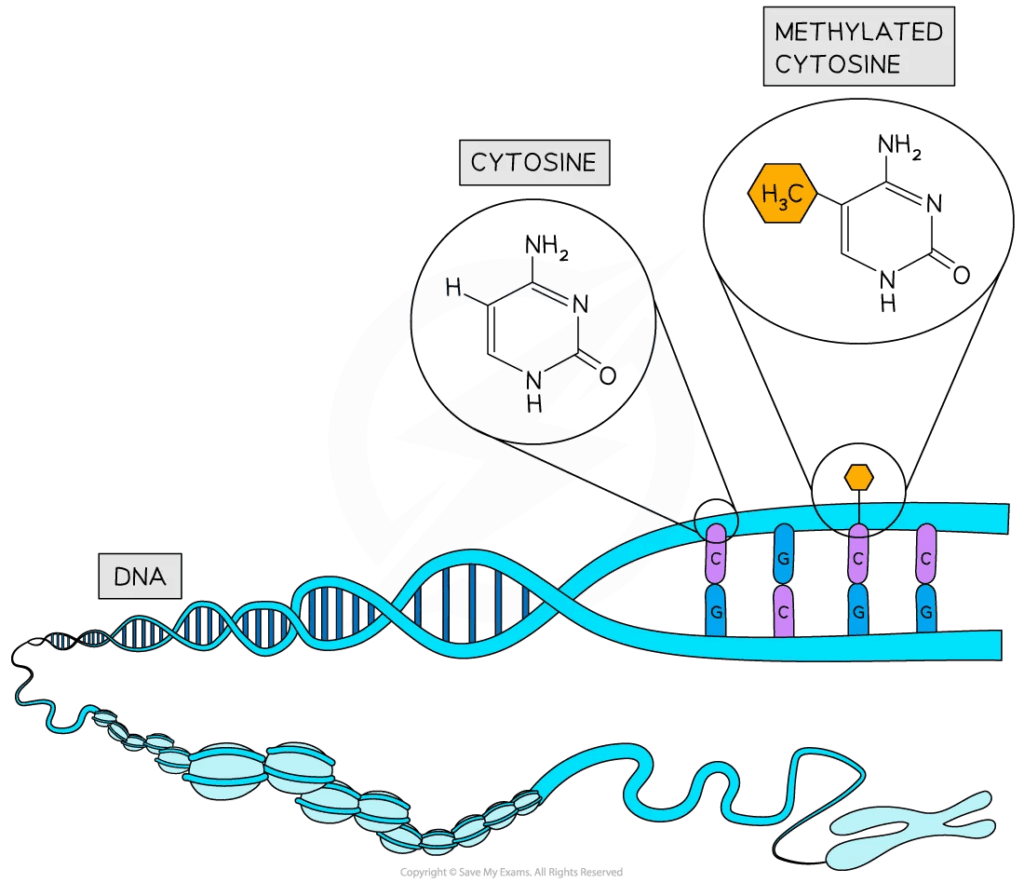
- Methyl groups added to cytosine bases (CpG islands).
- Methylation condenses chromatin, blocking transcription factor binding.
- Common in silencing repetitive DNA and transposable elements.
- Abnormal methylation patterns associated with cancer (silencing tumour suppressor genes).
- Methylation is maintained through cell division.
🧠 Examiner Tip: Epigenetics ≠ mutation. It’s about chemical modifications that affect expression without altering the base sequence.
📌 Histone Modification

- Histone tails undergo acetylation, methylation, phosphorylation, ubiquitination.
- Acetylation → loosens chromatin, increases transcription.
- Deacetylation → tightens chromatin, reduces transcription.
- Combinations of modifications form a “histone code.”
- Enzymes (HATs, HDACs) regulate these modifications.
🧬 IA Tips & Guidance: Research projects can analyse how environmental stress (e.g., salinity in plants) affects histone acetylation patterns using published datasets.
📌 Chromatin Remodeling
- Epigenetic changes alter balance between euchromatin and heterochromatin.
- Euchromatin is transcriptionally active, heterochromatin is silent.
- Remodeling complexes move or restructure nucleosomes.
- This allows access for transcription factors when needed.
- Essential in developmental gene activation/repression.
🌐 EE Focus: An EE could investigate epigenetic regulation in model organisms — e.g., how nutrition affects methylation in bees (workers vs queens).
📌 Epigenetics in Development
- Epigenetic programming directs stem cells into different lineages.
- X-chromosome inactivation in females equalises gene dosage.
- Imprinting silences one parental allele in certain genes.
- Epigenetic memory maintains cell identity across divisions.
- Reprogramming occurs during gamete formation and early development.
❤️ CAS Link: Students could create a science communication project explaining epigenetics to the public with the metaphor of “switches and dimmers on genes.”
🌍 Real-World Connection: Epigenetic drugs (HDAC inhibitors, DNMT inhibitors) are used in cancer therapy. Epigenetics explains why identical twins can differ in disease susceptibility.
📌 Inheritance and Reversibility
- Epigenetic marks can be passed to daughter cells.
- Some marks escape erasure in gametes, leading to transgenerational effects.
- Diet, toxins, and stress can all induce reversible epigenetic changes.
- Shows environment–gene interactions at a molecular level.
- Offers therapeutic potential for modifying gene expression.
🔍 TOK Perspective: Epigenetics blurs nature vs nurture. TOK issue: To what extent can science separate genetic destiny from environmental influence?