D1.3.3 TECHNIQUES IN GENE EDITING CRISPR AND APPLICATIONS
📌Definition Table
| Term | Definition |
|---|---|
| Gene editing | Direct manipulation of an organism’s DNA to add, remove, or modify genes. |
| CRISPR-Cas9 | A gene-editing tool derived from bacterial defense systems, using guide RNA and Cas9 nuclease to cut DNA at specific sites. |
| Guide RNA (gRNA) | Synthetic RNA molecule that directs Cas9 to the correct DNA sequence. |
| Cas9 | Enzyme that makes double-strand cuts in DNA at gRNA-specified sites. |
| Off-target effects | Unintended DNA modifications caused by imprecise editing. |
📌Introduction
Gene editing has revolutionized molecular biology, enabling precise modifications of DNA sequences. Among the various methods developed, CRISPR-Cas9 has emerged as the most powerful due to its accuracy, efficiency, and simplicity. Adapted from bacterial immune systems, CRISPR technology allows researchers to target virtually any gene for knockout, correction, or insertion, with wide-ranging implications in medicine, agriculture, and biotechnology.
📌 Mechanism of CRISPR-Cas9 Editing
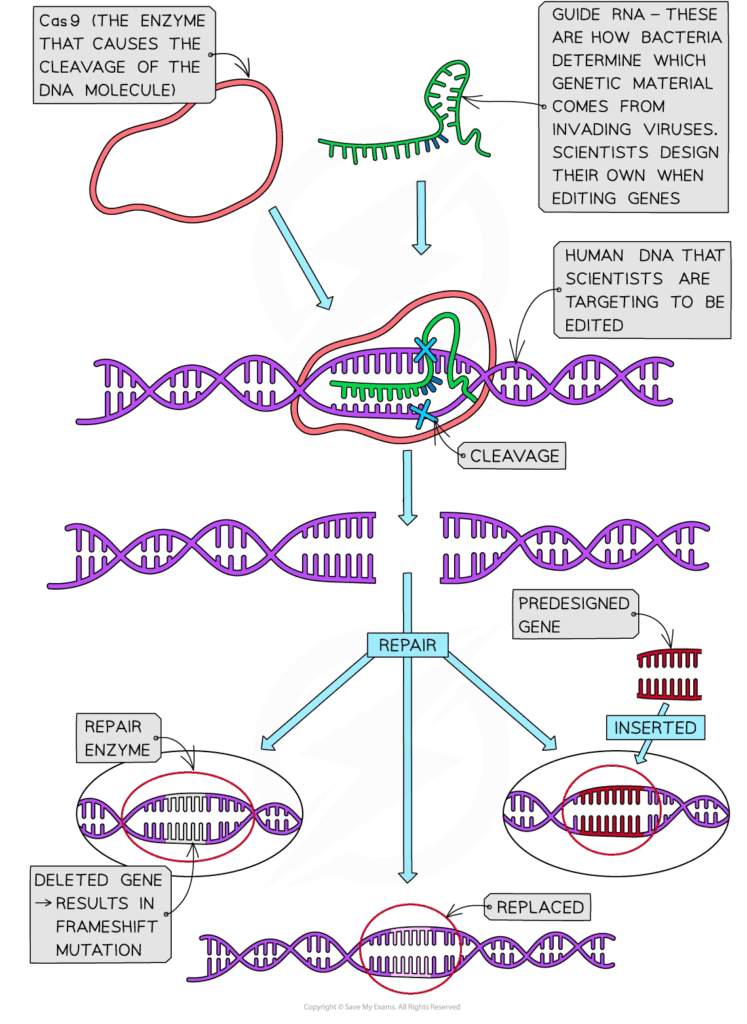
- A synthetic guide RNA (gRNA) binds to a specific DNA sequence via complementary base pairing.
- The Cas9 enzyme introduces a double-strand break at the targeted site.
- The cell repairs the break using one of two mechanisms:
- Non-homologous end joining (NHEJ): error-prone repair that often introduces mutations (useful for gene knockouts).
- Homology-directed repair (HDR): precise repair using a supplied DNA template for accurate gene correction or insertion.
- The result is targeted gene disruption or modification with high specificity.
🧠 Examiner Tip: Always distinguish between NHEJ (mutagenic, knockouts) and HDR (precise, knock-ins) when explaining CRISPR mechanisms.
📌 Applications in Medicine
- Correcting mutations responsible for genetic disorders (e.g., sickle-cell disease, cystic fibrosis).
- Engineering immune cells (CAR-T therapy) to fight cancers.
- Developing antiviral strategies by targeting viral DNA or RNA.
- Researching potential cures for polygenic diseases by editing multiple genes simultaneously.
- Advancing personalized medicine by tailoring gene edits to individual genomes.
🧬 IA Tips & Guidance: An IA could model CRISPR targeting using bioinformatics tools to identify PAM sequences in DNA, linking digital biology with molecular techniques.
📌 Applications in Agriculture and Biotechnology
- Creating crops resistant to pests, diseases, and environmental stress.
- Enhancing nutritional content (e.g., rice enriched with vitamins).
- Producing livestock with desirable traits such as faster growth or disease resistance.
- Industrial biotechnology uses gene editing to engineer microbes for biofuel or pharmaceutical production.
- Synthetic biology harnesses CRISPR to design novel organisms for environmental or medical use.
🌐 EE Focus: An EE could analyze ethical debates around CRISPR, investigating case studies such as gene-edited embryos, or explore agricultural applications and their impact on food security.
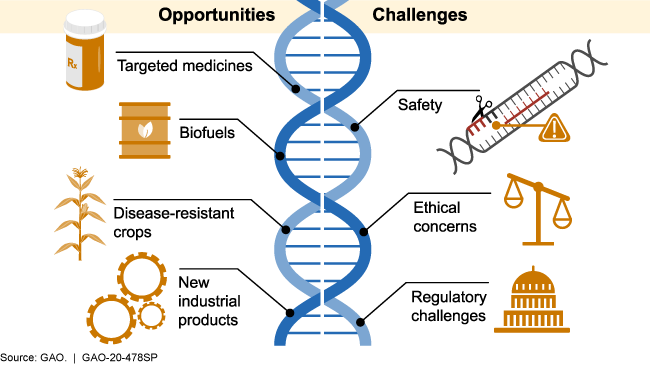
📌 Ethical, Safety, and Regulatory Issues
- Concerns about germline editing and heritable genetic modifications.
- Potential misuse in creating “designer babies.”
- Off-target mutations could cause unintended health risks.
- Ethical debates over editing animal and plant genomes for human benefit.
- Regulations vary worldwide, with stricter rules on human applications.
❤️ CAS Link: Students could hold a debate or awareness campaign on CRISPR ethics, engaging their peers in discussions about the balance between scientific progress and moral responsibility.
🌍 Real-World Connection: CRISPR is already in clinical trials for treating genetic diseases like sickle-cell anemia and beta-thalassemia. Agricultural scientists use it to engineer crops with greater yield and climate resilience, addressing food insecurity. However, controversies such as the creation of CRISPR-edited babies in China highlight ethical boundaries and global concerns. The technology stands at the intersection of innovation and responsibility, shaping the future of biology and medicine.
📌 Future Directions of Gene Editing
- Development of CRISPR variants (Cas12, Cas13) expands applications to RNA editing.
- Base editing and prime editing increase precision, reducing off-target risks.
- Potential for gene drives to alter wild populations, e.g., controlling malaria-spreading mosquitoes.
- Integration with AI and bioinformatics will improve guide RNA design.
- The challenge remains to balance medical promise with ethical safeguards.
🔍 TOK Perspective: CRISPR raises profound questions about the limits of human intervention. TOK reflection: If humans can rewrite the code of life, how do we determine what should or should not be changed? Does technological capability automatically justify its use?