C1.1.3 METABOLIC PATHWAYS AND ENZYME REGULATION
📌Definition Table
| Term | Definition |
|---|---|
| Metabolic pathway | A series of enzyme-catalyzed reactions that convert a starting molecule into a product. |
| Catabolism | Breakdown of complex molecules into simpler ones, releasing energy (e.g., respiration). |
| Anabolism | Synthesis of complex molecules from simpler ones, requiring energy (e.g., photosynthesis, protein synthesis). |
| Feedback inhibition | Regulation where the end-product of a pathway inhibits an earlier enzyme, preventing overproduction. |
| Allosteric regulation | Control of enzyme activity by binding of molecules to regulatory sites (not the active site), changing enzyme conformation. |
| Isoenzymes | Different enzymes that catalyze the same reaction but may differ in regulation or conditions of activity. |
| Metabolite | Intermediate product formed during a metabolic pathway. |
📌Introduction
Metabolism is the sum of all biochemical reactions in an organism. These reactions are highly organized into pathways, ensuring efficiency and control. Pathways can be linear (glycolysis), cyclic (Krebs cycle), or branched (amino acid biosynthesis). Enzymes play key roles in controlling these pathways, allowing cells to regulate energy supply, adapt to environmental changes, and maintain homeostasis. Without regulation, metabolic chaos would occur, wasting energy and resources.
📌 Organization of Metabolic Pathways
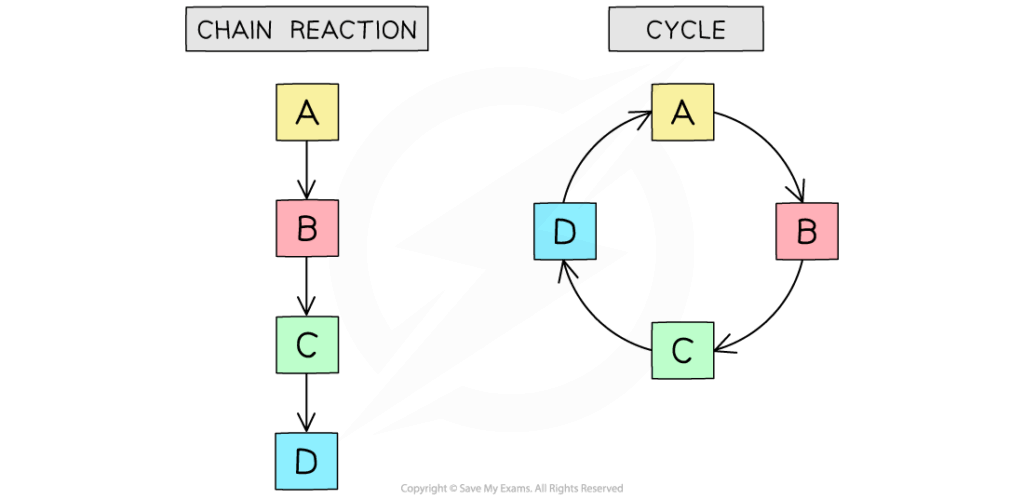
- Linear pathways: substrates converted step-by-step into final products (e.g., glycolysis converting glucose to pyruvate).
- Cyclic pathways: regenerate initial substrate with each turn (e.g., Krebs cycle, Calvin cycle).
- Branched pathways: intermediates serve as entry/exit points for multiple products (e.g., amino acid biosynthesis).
- Compartmentalization (e.g., mitochondria, chloroplasts, cytoplasm) allows separation of catabolic and anabolic pathways, preventing interference.
🧠 Examiner Tip: Examiners often ask for named examples of pathways. Always link structure (linear, cyclic) to function with specific cases like glycolysis or Krebs cycle.
📌 Catabolic vs Anabolic Pathways
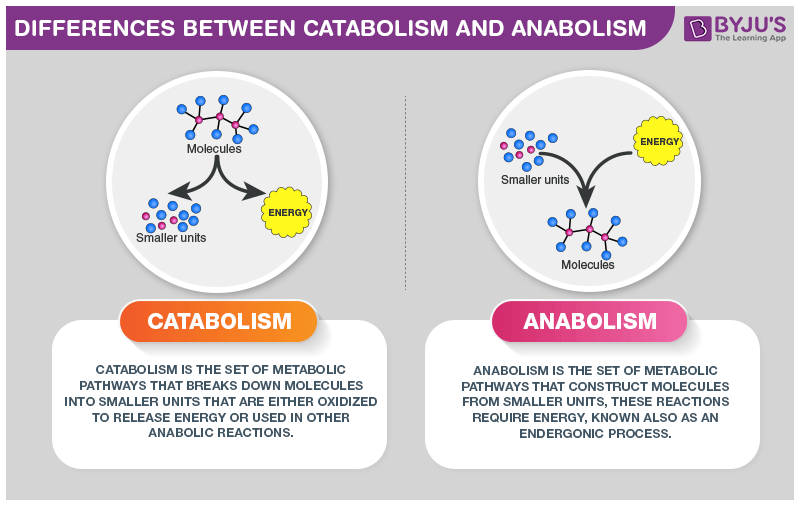
- Catabolic pathways:
- Break down complex molecules (polysaccharides, lipids, proteins).
- Release energy stored in chemical bonds.
- Example: cellular respiration (glucose → ATP).
- Anabolic pathways:
- Build complex molecules from simple precursors.
- Require energy input, often ATP and reducing power (NADPH).
- Example: photosynthesis, protein synthesis, glycogen synthesis.
- These pathways are interconnected, with catabolism providing energy for anabolism.
🧬 IA Tips & Guidance: Students could investigate enzyme regulation in respiration by measuring CO₂ output in yeast under varying glucose concentrations. Alternatively, experiments with inhibitors (e.g., cyanide on respiration) demonstrate pathway control.
📌 Enzyme Regulation in Pathways
- Rate-limiting steps: often controlled by allosteric enzymes sensitive to product/substrate levels.
- Feedback inhibition:
- End-product inhibits first committed step.
- Example: isoleucine inhibits threonine deaminase in amino acid synthesis.
- Covalent modification: phosphorylation/dephosphorylation alters enzyme activity.
- Allosteric activation: presence of a metabolite enhances enzyme activity.
- Regulation ensures efficiency and prevents accumulation of intermediates.
🌐 EE Focus: An EE could explore computational modeling of metabolic networks, or experimentally analyze how nutrient availability alters enzyme activity in fermentation vs aerobic respiration.
📌 Integration of Pathways in Cells
- Energy carriers (ATP, NADH, FADH₂, NADPH) link catabolic and anabolic reactions.
- Respiration: glycolysis, Krebs cycle, and oxidative phosphorylation integrate for maximum ATP yield.
- Photosynthesis: light-dependent reactions provide ATP and NADPH for the Calvin cycle.
- Amino acid, lipid, and carbohydrate metabolism are interconnected through shared intermediates (e.g., acetyl-CoA, pyruvate).
❤️ CAS Link: Students could design nutrition awareness campaigns showing how metabolic pathways explain balanced diets (carbohydrates, fats, proteins) and energy supply, linking biochemistry to public health.
🌍 Real-World Connection: Disruption of metabolic pathways leads to disease (e.g., PKU from defective phenylalanine metabolism, diabetes from impaired glucose regulation). Drugs often target metabolic enzymes (statins inhibit cholesterol synthesis, methotrexate blocks nucleotide synthesis). In biotechnology, metabolic engineering redirects pathways for biofuel or pharmaceutical production.
📌 Systems-Level View of Metabolism
- Metabolism is highly interconnected; altering one pathway affects many others.
- Systems biology uses computational models to map metabolic fluxes.
- Isotopic labeling (e.g., C¹⁴ glucose) helps trace metabolites in pathways.
- These approaches show metabolism as a network, not isolated chains of reactions.
🔍 TOK Perspective: Metabolic pathways are human-constructed models. TOK reflection: To what extent do pathway diagrams represent reality, or are they simplifications of a far more complex biochemical network?